Human brain cells developed in lab, grow in mice
A key type of human brain cell developed in the laboratory grows seamlessly when transplanted into the brains of mice, UC San Francisco researchers have discovered, raising hope that these cells might one day be used to treat people with Parkinson's disease, epilepsy, and possibly even Alzheimer's disease, as well as and complications of spinal cord injury such as chronic pain and spasticity.
"We think this one type of cell may be useful in treating several types of neurodevelopmental and neurodegenerative disorders in a targeted way," said Arnold Kriegstein, MD, PhD, director of the Eli and Edythe Broad Center of Regeneration Medicine and Stem Cell Research at UCSF and co-lead author on the paper.
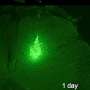
The researchers generated and transplanted a type of human nerve-cell progenitor called the medial ganglionic eminence (MGE) cell, in experiments described in the May 2 edition of Cell Stem Cell. Development of these human MGE cells within the mouse brain mimics what occurs in human development, they said.
Kriegstein sees MGE cells as a potential treatment to better control nerve circuits that become overactive in certain neurological disorders. Unlike other neural stem cells that can form many cell types—and that may potentially be less controllable as a consequence—most MGE cells are restricted to producing a type of cell called an interneuron. Interneurons integrate into the brain and provide controlled inhibition to balance the activity of nerve circuits.
To generate MGE cells in the lab, the researchers reliably directed the differentiation of human pluripotent stem cells—either human embryonic stem cells or induced pluripotent stem cells derived from human skin. These two kinds of stem cells have virtually unlimited potential to become any human cell type. When transplanted into a strain of mice that does not reject human tissue, the human MGE-like cells survived within the rodent forebrain, integrated into the brain by forming connections with rodent nerve cells, and matured into specialized subtypes of interneurons.
These findings may serve as a model to study human diseases in which mature interneurons malfunction, according to Kriegstein. The researchers' methods may also be used to generate vast numbers of human MGE cells in quantities sufficient to launch potential future clinical trials, he said.
Kriegstein was a co-leader of the research, along with Arturo Alvarez-Buylla, PhD, UCSF professor of neurological surgery; John Rubenstein, MD, PhD, UCSF professor of psychiatry; and UCSF postdoctoral scholars Cory Nicholas, PhD, and Jiadong Chen, PhD.
Nicholas utilized key growth factors and other molecules to direct the derivation and maturation of the human MGE-like interneurons. He timed the delivery of these factors to shape their developmental path and confirmed their progression along this path. Chen used electrical measurements to carefully study the physiological and firing properties of the interneurons, as well as the formation of synapses between neurons.
Previously, UCSF researchers led by Allan Basbaum, PhD, chair of anatomy at UCSF, have used mouse MGE cell transplantation into the mouse spinal cord to reduce neuropathic pain, a surprising application outside the brain. Kriegstein, Nicholas and colleagues now are exploring the use of human MGE cells in mouse models of neuropathic pain and spasticity, Parkinson's disease and epilepsy.
"The hope is that we can deliver these cells to various places within the nervous system that have been overactive and that they will functionally integrate and provide regulated inhibition," Nicholas said.
The researchers also plan to develop MGE cells from induced pluripotent stem cells derived from skin cells of individuals with autism, epilepsy, schizophrenia and Alzheimer's disease, in order to investigate how the development and function of interneurons might become abnormal—creating a lab-dish model of disease.
One mystery and challenge to both the clinical and pre-clinical study of human MGE cells is that they develop at a slower, human pace, reflecting an "intrinsic clock". In fast-developing mice, the human MGE-like cells still took seven to nine months to form interneuron subtypes that normally are present near birth.
"If we could accelerate the clock in human cells, then that would be very encouraging for various applications," Kriegstein said.