Pricey new blood thinner might be safer for leg clots
(HealthDay)—The new pill Eliquis prevents dangerous blood clots in the legs and lungs as well as standard therapy, though with less risk of serious bleeding, a new study shows.
The research, published online July 1 in the New England Journal of Medicine, may point doctors toward a simpler, if more costly, way to prevent repeat blood clots in patients at risk for venous thromboembolism.
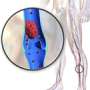
Venous thromboembolism includes two related conditions: deep vein thrombosis (DVT) and pulmonary embolism. Together, these conditions hospitalize more than 500,000 adults each year in the United States, according to the government's National Hospital Discharge Survey.
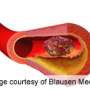
In DVT, a blood clot forms in the deep veins of the leg, causing swelling, redness, warmth and pain. If the blood clot breaks free, it can travel and lodge near the brain, heart or another vital organ, causing severe damage. If a clot blocks a blood vessel in the lungs, it's a life-threatening emergency called pulmonary embolism (PE). PEs are the third leading cause of cardiovascular death after heart attacks and strokes.
Blood clots in veins can happen without warning, but certain factors increase a person's risk including obesity, high blood pressure, long-distance travel, air pollution, cigarette smoking, pregnancy or recent surgery or injury.
Once a person has had one venous clot, they're more likely to get another, so doctors will often prescribe medication to lower the risk.
For years, the medication doctors relied on to prevent blood clots was a drug called warfarin, which is also sold under the brand name Coumadin.
Warfarin works well, but it's also tricky to take. Patients on the drug need regular blood tests—these can be weekly at the start of treatment—to make sure they're taking the right dose. And the dose can change from day to day or week to week. There are also a number of foods and drugs that can interact with warfarin, interfering with its effectiveness.
"It's really challenging for elderly patients to get it all right," said senior study author Dr. Jeffrey Weitz, a professor of medicine at McMaster University in Ontario, Canada.
In December, the U.S. Food and Drug Administration approved the drug Eliquis. Like warfarin, Eliquis prevents blood clots, but it works in a slightly different way than the older medication. It also doesn't require regular blood tests or changing dosages, making it much easier to manage.
One drawback of Eliquis is its price. Mail-order pharmacies charge between $250 and $275 for a 30-day supply of the medication in the United States, according to the website pharmacychecker.com. Warfarin, on the other hand, is $4 for a 30-day supply at stores like Target and Walmart. That means that one Eliquis pill costs about as much as an entire month's supply of warfarin.
For the new study, researchers compared Eliquis to warfarin in nearly 5,400 patients with a history of venous thromboembolism. The average age of study participants was 57. Roughly 60 percent were men. Sixty-five percent had a history of DVT. About 25 percent had a history of PE. About 9 percent had both DVT and PE.
Half of the study participants took 10 milligrams (mg) of Eliquis twice daily for seven days, before dropping their dosage to 5 mg twice daily for six months. The other half started with twice daily injections of the blood-thinning drug Lovenox (low-weight heparin), followed by daily, personalized warfarin therapy.
After six months, 59 patients in the Eliquis group and 71 patients who got standard therapy had a new blood clot. Of those, 12 patients in the Eliquis group and 15 patients in the warfarin group died from clots, showing that both drugs worked about equally well.
But patients who took Eliquis had less serious bleeding than those who took warfarin. Out of 2,676 patients taking Eliquis, 15 had major bleeding on the drugs. Of 2,689 patients taking warfarin, 49 experienced major bleeding.
"That's almost a 70 percent reduction in major bleeding with Eliquis, compared to conventional therapy. That's huge," said Weitz.
Beyond serious bleeding, Weitz said people taking Eliquis also had less nuisance bleeding of the gums or nose, which can lead patients to stop taking their medication.
"I think it's very important," said Weitz, who reported that he has been a consultant for study sponsors Bristol-Myers Squibb and Pfizer, along with other pharmaceutical companies, within the past three years.
Another expert who wasn't involved in the study agreed.
"This new approach may simplify the treatment regimen, improve patient convenience and substantially increase the safety of venous thromboembolism treatment, making this an attractive new option," said Dr. Gregg Fonarow, who is co-director of the University of California, Los Angeles, preventive cardiology program at the David Geffen School of Medicine.
More information: For more on venous thromboembolism, head to the U.S. National Heart, Lung, and Blood Institute.
Health News
Copyright © 2013