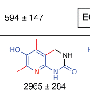
FDA warns of rare skin reactions to acetaminophen
(HealthDay)—The widely used painkiller acetaminophen, best known as Tylenol, can cause rare but serious skin reactions and a warning about this danger will be added to product labels, the U.S. Food and Drug Administration said Thursday.
Acetaminophen is also often used in combination with other medicines, including opioids for pain and medicines to treat colds, coughs, allergy, headache and sleeping problems.
According to the FDA, acetaminophen can trigger three serious skin reactions. Two of them—Stevens-Johnson Syndrome and toxic epidermal necrolysis—usually require hospitalization and can be lethal.
The reactions usually begin with flu-like symptoms followed by rash, blistering and extensive damage to the skin surface. Recovery can take weeks or months, and possible complications include scarring, skin color changes, blindness and damage to internal organs.
A third skin reaction that can be cause by acetaminophen is called acute generalized exanthematous pustulosis. It usually resolves within two weeks after a patient stops taking acetaminophen.
People who develop a rash or other skin reaction while taking acetaminophen should stop taking the drug and seek immediate medical attention, the FDA said.
"This new information is not intended to worry consumers or health care professionals, nor is it meant to encourage them to choose other medications," Dr. Sharon Hertz, deputy director of FDA's Division of Anesthesia, Analgesia and Addiction, said in an agency news release. "However, it is extremely important that people recognize and react quickly to the initial symptoms of these rare, but serious, side effects, which are potentially fatal."
The FDA said that a warning about these skin reactions will be added to the labels of all prescription medicines containing acetaminophen, and the agency will work with manufacturers to have warnings added to the labels of non-prescription medicines that contain acetaminophen.
The FDA's decision to add warnings about possible skin reactions to products with acetaminophen is based on an analysis of data showing that there were 107 cases of acetaminophen-related skin reactions in the U.S. between 1969 and 2012. These cases resulted in 67 hospitalizations and 12 deaths.
Other drugs used to treat fever and pain, such as nonsteroidal anti-inflammatory drugs (NSAIDs) including ibuprofen and naproxen, already carry warnings about the risk of serious skin reactions.
Two years ago, the FDA took steps to reduce the risk of liver damage from acetaminophen. The agency asked manufacturers of prescription products to limit acetaminophen to 325 milligrams per tablet or capsule and required all prescription acetaminophen products to include a boxed warning about liver damage risk.
Hertz stressed that the, "FDA's actions should be viewed within the context of the millions who, over generations, have benefited from acetaminophen. Nonetheless, given the severity of the risk, it is important for patients and health care providers to be aware of it."
More information: The U.S. National Library of Medicine has more about acetaminophen.
Health News
Copyright © 2013