August 20, 2013 report
How many types of neurons do we need to define?
(Medical Xpress)—A recent perspective paper published in Science has raised some important, and timely, questions regarding neural diversity. The authors, from Columbia, MIT, and New York University, would simply like to know how many kinds of neurons exist in the brain. For scientists who study glial cells, it may be enough to have a single named class of neuron, but the researchers here all study motorneurons of one kind or another. In particular, they are interested in treating neurodegenerative diseases, which often have clear motor deficits as their major pathology. To treat these diseases, researchers are attempting to differentiate embryonic stem cells (ESCs) into particular cell subtypes that could be used to restore normal function. As the authors observe, it would be handy if we could constrain the enormous range of biochemical, morphological and electrophysiological peculiarities that neurons display, into well-defined categories that could be referred to by name.
The reality is that we cannot have a simple neuron taxonomy like we do, for example, with animals. Occasional hybrids aside, animal species are those that can produce fertile offspring when they mate. Name proliferation in neuroscience is instead open-ended, and the rate at which it occurs is its major parameter. It seems that we have moved beyond the age of the Neuro Rock Star, and honorifics might no longer be expected. While only a few additions to the classics—like the Betz cells of the motor cortex, Martinotti cells, and Cajal-Retzius cells—have managed to gain penetrance in neuroculture, there has been an explosion in potential ways to define neurons in terms of the kinds of genes and proteins that they are geared up to produce. What then is a suitable way to tame this new jungle?
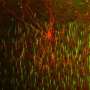
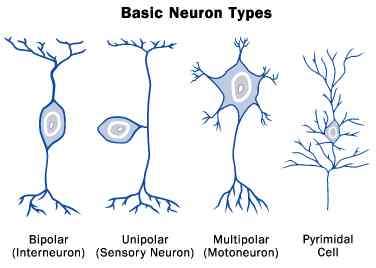
With an eye toward practicality, perhaps the best way forward is to stick with good old structure-function criteria, and now begin to concentrate on the function part. While genes and proteins can be manipulated ad nauseum and shown to have various effects on the morphology of neurons, we still lack a good understanding of what taking on one of those few basic cell plans, as shown in the familiar picture above, can do for a cell in terms of function. If we are concerned about restoring function to diseased brains on a cell by cell basis, then lets ask a few questions not about how molecular players generate morphology, but rather what the function of that fairly familiar morphology might be, and use that as a basis for future name derivations.
In other words, without an understanding of why cell shape slides along the continuum from bipolar, to pseudo-unipolar, to multipolar or other variations on the near-far/dendrite-axon dichotomy, to meet functional energetic needs, purely biochemical naming schemes will remain largely sterile. We may know, for example, that that the main process of a unipolar neuron stands off of the cell body a few microns, while that of the bipolar cell runs through it, and that a spike may slow down a little in the latter, but without knowing the functional implications of swapping one for the other, we actually know very little.
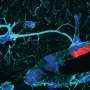
For the case then of the motorneuron, as it is dear to the authors, we might ask the following question. Why did our predecessors begin, or continue, to use acetlycholine at their neuromuscular junctions, while the branch that led from our nearest common ancestor with invertebrates, to their modern day insect or crustacean counterparts, continued to employ glutamate, and the synaptic optimizations therein at this contact? The neuromuscular junction (NMJ) is the membranous rendezvous made between the motorneuron and its target muscle. It is undoubtedly the most studied of all cell-type specific synapses, and yet still this basic question, likely asked before, remains unanswered. Many variables, including speed vs energy efficiency of synaptic transmission, vulnerability to attack by toxin, or ease of glial membrane investment vs ease of access by 3rd-party gaba-spritzing processes that join the junctional triad to provide additional modulation, may in fact all play a significant role here.
It has emerged that the preference for one transmitter or another, like that for acetylcholine or glutamate at the NMJ, is not always as clear-cut as we had previously assumed. Cells and synapses may progress through somewhat predictable developmental phases where one or another transmitter is favored, but often significant co-loading and release can be found as a stable state for the synapse. If biochemistry then is to be one guide to naming neurons, the effects of possessing a particular biochemistry should formulate the description. If, for example, a transmitter-like histamine acts basically like an irritant, other cells may essentially seek to see its release inhibited. The cell that chooses to manufacture histamine might need to resort to it in the absence of say, a robust cytoskeleton, or sufficient mitochondria and ion pumping mechanisms to maintain turgor to resist the expansive push of competing synapses.
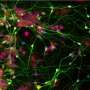
The authors have pointed to previous papers that have completed exhaustive analyses of particular brain structures, and the cell types within them. For the retina, one study proposed that since each cell type expressed a unique set of transcription factors, they could be used to generate a "barcode" for that cell type. That may be useful for some classification purposes, but if we are now at the level of basically saying, "lets pop in a replacement cell module to deep layer motor cortex," the useful descriptors, in terms of survivability and integration, may not yet be detailed barcodes. Instead they might be designations that pertain to how well the cells in the module get on with each other and the new the neighbors, or perhaps more specifically, help complete local metabolic circuits. For example, we saw recently that when human astrocytes were transplanted into rat cortex they flourished, and nourished, the local environment in such a way that they did not crowd out the much smaller host cell astrocytes, and managed to hold their own in this new environment.
Transplantation researchers have long understood that success ultimately depends on a favorable outcome of the competition between cell types, particularly when control of the immune system is at stake due to graft of foreign tissue. But even the individual organism is a mosaic of mother and father specific gene patterns with whole organs, or at least parts of organs, tending to be dominated by genes originating from one or the other. For the brain itself, substructures and cell types within them may come to be understood in terms higher level characteristics which take into consideration parental origin and particular epigenetic factors. As for coming up with names or classification schemes, perhaps it is still best to let principles emerge as function becomes better understood. The alternative, seeking to apply esoteric statistical methods or classifiers to unbounded possible molecular descriptors which are themselves of limited reliability, may provide more noise than signal when it comes to naming neurons.
More information: Mapping Neuronal Diversity One Cell at a Time, Science 16 August 2013: Vol. 341 no. 6147 pp. 726-727. DOI: 10.1126/science.1235884
Abstract
How many types of nerve cells are there in the mammalian central nervous system (CNS)? We still do not have a satisfactory answer to this deceptively simple question, and yet the precise assignment of nerve cells to well-defined subtype categories is critical both for elucidating the function of neural circuits and for the success of neural regenerative medicine. Amid the anatomical, electrophysiological, and biochemical diversity of nerve cells, the field is struggling to devise simple and clear criteria for neuronal classification. A universally applicable classification system should be based on traits that are objectively quantifiable, sufficiently diverse, and reproducible in independent laboratories. Such a classification method would provide new insights into CNS organization, development, and function, and might reveal unexpected relationships between neuronal subtypes.
© 2013 Medical Xpress