Scientists use drug to repair rare birth defect
University of British Columbia and Vancouver Coastal Health scientists have developed a potential cure for a rare eye disease, showing for the first time that a drug can repair a birth defect.
They formulated the drug Ataluren into eye drops, and found that it consistently restored normal vision in mice who had aniridia (ANN-uh-ridee- uh), a condition that severely limits the vision of about 5,000 people in North America. A small clinical trial with children and teens is expected to begin next year in Vancouver, the U.S. and the U.K.
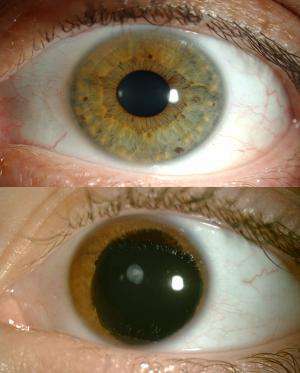
Aniridia is caused by the presence of a "nonsense mutation" – an extra "stop sign" on the gene that interrupts production of a protein crucial for eye development. Aniridia patients don't have an iris (the coloured ring around the pupil), and suffer many other eye abnormalities.
Ataluren is believed to have the power to override the extra stop sign, thus allowing the protein to be made. The UBC-VCH scientists initially thought the drug would work only in utero – giving it to a pregnant mother to prevent aniridia from ever arising in her fetus. But then they gave their specially formulated Ataluren eye drops, which they call START, to two-week-old mice with aniridia, and found that it actually reversed the damage they had been born with.
"We were amazed to see how malleable the eye is after birth," said Cheryl Gregory-Evans, associate professor of ophthalmology and visual sciences and a neurobiologist at the Vancouver Coastal Health Research Institute. "This holds promise for treating other eye conditions caused by nonsense mutations, including some types of macular degeneration. And if it reverses damage in the eye, it raises the possibility of a cure for other congenital disorders. The challenge is getting it to the right place at the right time."
BACKGROUND: A POTENTIAL CURE FOR ANIRIDIA
Bad vision at birth, worse vision later: Aniridia is apparent at birth because of the missing iris. Toddlers with aniridia need eyeglasses to see, sunglasses or darkened contact lenses to protect their eyes from overexposure to light, and cannot read small text. Their eyes are continually moving, making it difficult for them to focus, and have higher internal pressure (glaucoma), which damages the optic nerve as they get older. They are also prone to corneal damage in their teens and early adulthood. Eventually, most people with aniridia are considered legally blind, and must resort to Braille or expensive electronic aids to read.
The plasticity of the eye: The reversal of tissue damage in young mice, published online today by the Journal of Clinical Investigation, fits with the fact that mammals' eyes aren't fully formed at birth. Human babies don't discern colours until they are six months old, and their depth perception isn't fully developed until the age of five.
Nonsense suppressor: Ataluren, made by the New Jersey-based PTC Therapeutics, is thought to be a "nonsense suppressor" – it silences the extra "stop codon" on the gene and allows a complete protein to be assembled. The drug is currently being tested as a treatment for cystic fibrosis and Duchenne muscular dystrophy, which are also caused by nonsense mutations.
A gritty solution: Gregory-Evans' first attempt at creating Ataluren eye drops proved unsuccessful. The drug didn't dissolve, and thus irritated the mice's eyes. So she turned to Kishor Wasan, a professor and associate dean in the Faculty of Pharmaceutical Sciences, who ground the drug into a very fine powder and combined it with a solution that adhered better to the eye.
A multidisciplinary team: Gregory-Evans also collaborated with her husband, Kevin Gregory-Evans, the Julia Levy BC Leadership Chair in Macular Research and an ophthalmologist at the VCH Eye Care Centre, who treats B.C. patients with aniridia. He administered the vision tests for the mice used in the study.
Clinical trial: The forthcoming clinical trial, involving about 30 patients, will be led by Gregory-Evans in Vancouver, and is being supported by the Vision for Tomorrow Foundation, a U.S.-based charity focused on aniridia and albinism (an absence of pigmentation in skin, hair and eyes that results in poor vision). If START is proven to be safe and effective, children with aniridia would use the drops twice a day for the rest of their lives. The drug would probably not reverse the condition in adults because their eyes would already be damaged beyond repair.