Scientists crack riddle of important drug target
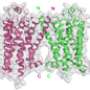
A new approach to mapping how proteins interact with each other, developed at the Salk Institute for Biological Studies, could aid in the design of new drugs for diseases such as diabetes and osteoporosis. By reengineering proteins using artificial amino acids, the Salk scientists determine the detailed molecular structure of a cellular switch and its ligand, the molecule that turns it on. The switch—corticotrophin releasing factor type 1 (CRF1R)—belongs to a class of cellular receptors whose structures are notoriously hard to determine. These receptors regulate processes throughout the body and are involved in many diseases.
"Only when you know how the ligand binds to the receptor can you design drugs that target these processes," says senior study author Lei Wang, an associate professor in Salk's Jack H. Skirball Center for Chemical Biology and Proteomics and holder of the Frederick B. Rentschler Developmental Chair. Wang and his team describe the new structure and method in a Cell paper published online November 27.
Typically, researchers determine the three-dimensional arrangement of atoms in a protein molecule by crystalizing the protein and measuring how x-rays diffract off the crystals. But the receptor class the Salk scientists studied—known as class B G-protein coupled receptors (GPCRs)—are tricky to coax into crystal form, since they are only stable when embedded in the cellular membranes that enclose a cell's cytoplasm and nucleus. As a result, getting a complete picture of their structure—let alone the structure of the receptor combined with its bound ligand—hasn't been possible.
Wang's team turned to a new approach to try and figure out what CRF1R's binding pocket—the area where the ligand attaches—looked like. Using genetic engineering, the scientists added a unique new amino acid, one of the building blocks of proteins, to spots all along CRF1R.
"When you shine light on this artificial amino acid, it grabs nearby molecules," explains Irene Coin, a postdoctoral fellow in Wang's team. "It's like a sticky hand."
When the artificial amino acid, Azi, was added to any spot where the CFR1R ligand attached to the receptor, the sticky hand grabbed the ligand, a molecule called urocortin-1, and kept it bound to the receptor. If Azi was integrated into a place where urocortin-1 didn't associate, however, it would have nothing to grab. By detecting whether CFR1R and urocortin-1 become irreversibly attached, the researchers would know whether the Azi had been integrated into part of the binding pocket or not.
Repeating this technique throughout the CFR1R molecule revealed that the receptor's binding pocket consisted of at least 35 amino acids. But that didn't give the researchers the full picture they wanted of the interaction.
"This first, sticky hand probe had given us information about the shape of the binding pocket," says Wang. "But we still didn't know how the ligand is oriented inside that pocket."
So they used a second probe—one which was more selective than the "sticky hand" in the receptor. This time, the probe would only capture one particular amino acid—cysteine. "We inserted cysteines along the ligand to figure out which parts of the receptor were close to precise spots of the ligand", says Wang. It took more than a hundred different combinations to get a perfect match, where the artificial amino acids in the receptor lined up with the cysteines in the urocortin-1.
"We discovered that the ligand lies in the receptor's binding pocket like a too-tall person in a bathtub," Coin says. "One end of the ligand is like the head sticking out, and on the other end, the feet are dangling out."
"This shape makes sense in light of previous data," says Wang. "Because we already knew that you can add a lot of molecules onto the feet of the ligand and it doesn't affect the receptor-ligand interaction."
When they know which amino acids in a ligand interact with which amino acids in a receptor, scientists can begin designing ways to block the interaction between the pair, by creating new molecules that attach to the receptor, for instance. So Wang sees the new structure—and the new approach for determining it-as a key step forward for designing drugs that target class B GPCRs.
"The beauty of this method is that it can be a general method to map other binding pockets and protein interactions as well," Wang adds. "Its use isn't limited to only GPCRs."