Stent to treat pancreatic cysts approved
(HealthDay)—The Axios Stent and Delivery System has been approved by the U.S. Food and Drug Administration to treat infected pancreatic cysts that won't drain on their own and could become life threatening, the FDA said in a news release.
The pancreas, found in the upper abdomen behind the stomach, produces insulin that helps regulate blood sugar and helps digestion. If pancreatic ducts become blocked from gallstones or injury, enzymes that back up into the organ can cause formation of pancreatic pseudocysts.
Most resolve on their own, but some become infected and can lead to a life-threatening blood infection, the FDA said.
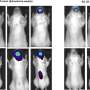
The Axios stent is a wire mesh tube that can expand to more than 1/2-inch diameter. It was evaluated among 33 clinical study participants who had a pancreatic pseudocyst at least six centimeters in diameter. Some 86 percent of pseudocysts treated shrank by half or more, the FDA said.
The most common side effects of the stent included abdominal pain, nausea and vomiting.
The device is produced by Xlumena Inc. of Mountain View, Calif.
More information: Visit Medline Plus to learn more about pancreatic pseudocysts.
Copyright © 2013 HealthDay. All rights reserved.