Chemical signaling simulates exercise in cartilage cells
Cartilage is notoriously difficult to repair or grow, but researchers at Duke Medicine have taken a step toward understanding how to regenerate the connective tissue. By adding a chemical to cartilage cells, the chemical signals spurred new cartilage growth, mimicking the effects of physical activity.
The findings, published online in the Proceedings of the National Academy of Sciences the week of Jan. 13, 2014, point to an ion channel called TRPV4 as a potential target for new therapies to treat osteoarthritis or even regrow cartilage.
Articular cartilage is the tissue that lines joints such as hips, knees and shoulders, providing cushioning and smooth movement. Similar to bones and muscles, cartilage only stays healthy and strong through loading, or applying force, through physical activity.
Abnormal forces on the joints can cause a variety of problems leading to pain and loss of mobility. Overloading joints through overuse or injury can lead to the cartilage breaking down, while lack of use can result in cartilage wasting through atrophy. Both kinds of cartilage deterioration leave joints prone to osteoarthritis, a degenerative and debilitating disease.
Until recently, researchers did not know how cartilage converts mechanical loading into the ion channel signals that promote growth. Understanding how cartilage senses mechanical loading could equip researchers with the knowledge needed to prevent or better treat joint diseases.
"Mechanical loading plays a critical role in the overall health of the cartilage," said Farshid Guilak, Ph.D., Laszlo Ormandy Professor of Orthopaedic Surgery at Duke and the study's senior author. "If we can figure out how cartilage cells sense mechanical loads, we can trick them into thinking they are being exercised or stop them from responding to abnormal loading. Think of it as artificial exercise for your cartilage."
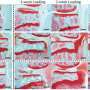
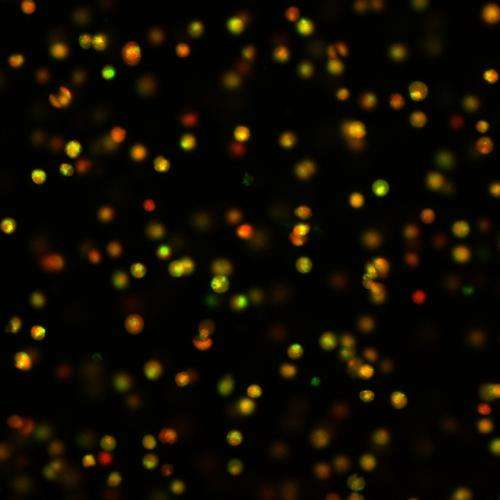
In the study, led by MD/PhD student Christopher O'Conor, the researchers looked at articular cartilage cells from pigs and focused on TRPV4, an ion channel abundant in cartilage cells that can be turned on during mechanical loading. When the researchers "exercised" the cartilage cells using mechanical loading, the cells sensed the loading and grew cartilage tissue. When they added a compound that blocked TRPV4, essentially turning off signals from the ion channel, the cartilage did not grow and the effects of the mechanical loading were lost.
Next, the researchers substituted mechanical loading for a chemical that activated TRPV4. Without having to exercise the cartilage, they observed the growth of cartilage even more so than with the mechanical loading. The findings suggest that TRPV4 is responsible for sensing mechanical loading in the cartilage.
Now that they know that turning on TRPV4 can simulate the effects of mechanical loading in cartilage cells, the researchers are looking at ways to harness this potential.
"Our next step is to see if this synthetic 'exercising' technology works on human cells that could be used to regrow new human cartilage," said O'Conor, who is completing his MD/PhD degree at the University of North Carolina at Chapel Hill and is performing his dissertation work with Guilak in the Duke Orthopaedic Bioengineering Laboratories.
Beyond growing new cartilage, the researchers will investigate whether the compounds that activate or block TRPV4 could act as new therapies to prevent cartilage degeneration and joint disease.
More information: TRPV4-mediated mechanotransduction regulates the metabolic response of chondrocytes to dynamic loading, www.pnas.org/cgi/doi/10.1073/pnas.1319569111