Scientists create detailed picture of protein linked to learning, pain and brain disorders
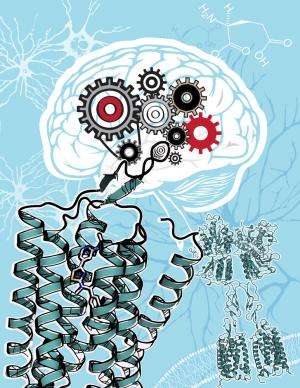
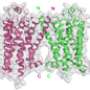
Researchers at The Scripps Research Institute (TSRI) and Vanderbilt University have created the most detailed 3-D picture yet of a membrane protein that is linked to learning, memory, anxiety, pain and brain disorders such as schizophrenia, Parkinson's, Alzheimer's and autism.
"This receptor family is an exciting new target for future medicines for treatment of brain disorders," said P. Jeffrey Conn, PhD, Lee E. Limbird Professor of Pharmacology and director of the Vanderbilt Center for Neuroscience Drug Discovery, who was a senior author of the study with Raymond Stevens, PhD, a professor in the Department of Integrative Structural and Computational Biology at TSRI. "This new understanding of how drug-like molecules engage the receptor at an atomic level promises to have a major impact on new drug discovery efforts."
The research—which focuses on the mGlu1 receptor—was reported in the March 6, 2014 issue of the journal Science.
A Family of Drug Targets
The mGlu1 receptor, which helps regulate the neurotransmitter glutamate, belongs to a superfamily of molecules known as G protein-coupled receptors (GPCRs).
GPCRs sit in the cell membrane and sense various molecules outside the cell, including odors, hormones, neurotransmitters and light. After binding these molecules, GPCRs trigger a specific response inside the cell. More than one-third of therapeutic drugs target GPCRs—including allergy and heart medications, drugs that target the central nervous system and anti-depressants.
The Stevens lab's work has revolved around determining the structure and function of GPCRs. GPCRs are not well understood and many fundamental breakthroughs are now occurring due to the understanding of GPCRs as complex machines, carefully regulated by cholesterol and sodium.
When the Stevens group decided to pursue the structure of mGlu1 and other key members of the mGlu family, it was natural the scientists reached out to the researchers at Vanderbilt. "They are the best in the world at understanding mGlu receptors," said Stevens. "By collaborating with experts in specific receptor subfamilies, we can reach our goal of understanding the human GPCR superfamily and how GPCRs control human cell signaling."
Colleen Niswender, PhD, director of Molecular Pharmacology and research associate professor of Pharmacology at the Vanderbilt Center for Neuroscience Drug Discovery, also thought the collaboration made sense. "This work leveraged the unique strengths of the Vanderbilt and Scripps teams in applying structural biology, molecular modeling, allosteric modulator pharmacology and structure-activity relationships to validate the receptor structure," she said.
The Challenge of the Unknown
mGlu1 was a particularly challenging research topic.
In general, GPCRs are exceedingly flimsy, fragile proteins when not anchored within their native cell membranes. Coaxing them to line up to form crystals, so that their structures can be determined through X-ray crystallography, has been a formidable challenge. And the mGlu1 receptor is particularly tricky as, in addition to the domain spanning the membrane, it has a large domain extending into the extracellular space. Moreover, two copies of this multidomain receptor associating in a dimer are needed to transmit glutamate's signal across the membrane.
The task was made more difficult because there was no template for mGlu1 from closely related GPCR proteins to guide the researchers.
"mGlu1 belongs to class C GPCRs, of which no structure has been solved before," said TSRI graduate student Chong Wang, a first author of the new study with TSRI graduate student Huixian Wu. "This made the project much harder. We could not use other GPCRs as a template to design constructs for expression and stabilization or to help interpret diffraction data. The structure was so different that old school methods in novel protein structure determination had to be used."
Surprising Results
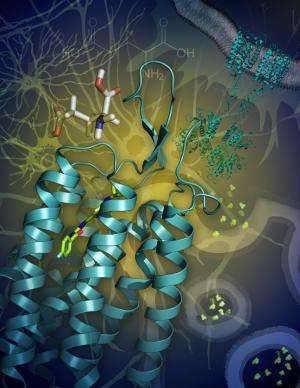
The team decided to try to determine the structure of mGlu1 bound to novel "allosteric modulators" of mGlu1 contributed by the Vanderbilt group. Allosteric modulators bind to a site far away from the binding site of the natural activator (in this case, presumably the glutamate molecule), but change the shape of the molecule enough to affect receptor function. In the case of allosteric drug candidates, the hope is that the compounds affect the receptor function in a desirable, therapeutic way.
"Allosteric modulators are promising drug candidates as they can 'fine-tune' GPCR function," said Karen Gregory, a former postdoctoral fellow at Vanderbilt University, now at Monash Institute of Pharmaceutical Sciences. "However, without a good idea of how drug-like compounds interact with the receptor to adjust the strength of the signal, discovery efforts are challenging."
The team proceeded to apply a combination of techniques, including X-ray crystallography, structure-activity relationships, mutagenesis and full-length dimer modeling. At the end of the study, they had achieved a high-resolution image of mGlu1 in complex with one of the drug candidates, as well as a deeper understanding of the receptor's function and pharmacology.
The findings show that mGlu1 possesses structural features both similar to and distinct from those seen in other GPCR classes, but in ways that would have been impossible to predict in advance.
"Most surprising is that the entrance to a binding pocket in the transmembrane domain is almost completely covered by loops, restricting access for the binding of allosteric modulators," said Vsevolod "Seva" Katritch, assistant professor of molecular biology at TSRI and a co-author of the paper. "This is very important for understanding action of the allosteric modulator drugs and may partially explain difficulties in screening for such drugs.
"The mGlu1 receptor structure now provides a solid platform for much more reliable modeling of closely related receptors," he continued, "some of which are equally important in drug discovery."
More information: "Structure of a class C GPCR metabotropic glutamate receptor 1 bound to an allosteric modulator," Science, 2014.