US approves novel remedy for hayfever, pollen allergies (Update)
US regulators on Wednesday approved a French-made remedy for alleviating symptoms of hayfever and certain allergies to grass pollen.
The tablet, called Oralair, dissolves under the tongue and must be taken daily for four months prior to the start of the grass pollen season, and then continued throughout the season.
Pre-approval trials on 2,500 people found that those who took the remedy experienced a 16 to 30 percent reduction in symptoms, which can include sneezing, runny nose, and itchy, watery eyes.
Manufactured by Stallergenes S.A. of Antony, France, the tablet contains freeze-dried extracts from the pollens of five grasses, including Kentucky Blue Grass, Orchard, Perennial Rye, Sweet Vernal and Timothy.
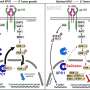
It is the first US Food and Drug Administration-approved allergen extract that can be dissolved under the tongue, the agency said in a statement.
"The approval of Oralair provides an alternative to allergy shots that must be given in a health care provider's office," said Karen Midthun, director of the FDA's Center for Biologics Evaluation and Research.
"While there is no cure for grass pollen allergies, they can be managed through treatment and avoiding exposure to the pollen."
These allergies affect some 30 million people in the United States and more than 500 million worldwide, according to the FDA.
© 2014 AFP