ALTTO test of dual HER2 blockade finds single agent remains the gold standard
In the largest clinical trial testing the effectiveness of one versus two drugs to treat HER2-positive breast cancer, lapatinib (Tykerb) did not add benefit to the standard trastuzumab (Herceptin) adjuvant therapy, researchers report at the 50th annual meeting of the American Society of Clinical Oncology (ASCO).
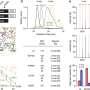
Results of the phase III clinical trial, ALTTO (Adjuvant Lapatinib and/or Trastuzumab Treatment Optimization study), demonstrated that adding lapatinib to trastuzumab and chemotherapy did not improve patient outcome (defined as disease-free survival or overall survival), and that use of lapatinib significantly increased toxicity.
"These findings suggest that standard adjuvant (post-surgery) treatment for early stage HER2-positive breast cancer should remain trastuzumab in combination with chemotherapy," says Edith A. Perez, M.D., deputy director at large of the Mayo Clinic Cancer Center, and director of the Breast Cancer Translational Genomics Program at Mayo Clinic in Florida.
"The ALTTO trial did not confirm the working hypothesis that adding lapatinib to trastuzumab, either concurrently or sequentially, would improve patient outcome (which was the primary endpoint)," she says. "These results were surprising and very important, as we had hypothesized and hoped that dual blockade of HER2 using two anti-HER2 agents would provide more benefit compared to single anti-HER2 therapy."
Dr. Perez added that the findings were additionally disappointing in that they did not confirm the benefit of using both anti-HER2 therapies predicted from the NeoALTTO clinical trial, which tested the therapies before cancer surgery (called neoadjuvant therapy). Some important positive results were seen in ALTTO, such as better outcome than expected for the overall group of patients, and a low risk of cardiac toxicity.
The 8,381-participant, 44-country phase III clinical trial was supported by The North American Breast Cancer Group (NABCG), based in the United States, and the Breast International Group (BIG) in Brussels, Belgium, the National Cancer Institute and Glaxo SmithKline. NABCG consists of six National Cancer Institute (NCI)-funded clinical trials cooperative groups. NCI is part of the National Institutes of Health.
Dr. Perez and Martine Piccart, M.D., Ph.D., professor of oncology at the Université Libre de Bruxelles, Belgium, are co-chairs of ALTTO. Dr. Piccart is lead investigator for BIG, which she founded in 1996. She presented results of NeoALTTO at the 2013 San Antonio Breast Cancer Symposium.
Dr. Perez predicts that the ALTTO results will be far-reaching in that they suggest that improved pathological complete response (pCR) in neoadjuvant clinical trials of patients with HER2-positive breast cancer may "not be predictive of improved long-term outcome of specific treatment in the adjuvant setting."
When therapy is given before surgery (called neoadjuvant therapy), pathologists can examine excised breast tissue for evidence of pCR—complete absence of cancer. Researchers and physicians have wondered, however, if pCR after neoadjuvant treatment is indeed linked to better outcomes for early stage HER2-positive breast cancer.
"The pCR measure from neoadjuvant clinical trials is increasingly being used as a surrogate marker of outcome in adjuvant studies. The results of ALTTO suggest that pCR is not a factor that reliably predicts the long-term benefit when comparing two treatments," Dr. Perez says.
"The fact is that these ALTTO adjuvant results call into question the entire way breast cancer care and research have been leaning in the last few years—which is to rely on pCR from neoadjuvant studies as surrogates of long-term outcome," she says. "We believe these results will be difficult for the field to absorb, but will ultimately guide our clinical research."
Specific findings from ALTTO will be presented at ASCO.