Team explains how stiffness in breast tissue contributes to invasive carcinoma
A team of researchers led by David J. Mooney, Robert P. Pinkas Family Professor of Bioengineering at the Harvard School of Engineering and Applied Sciences, have identified a possible mechanism by which normal cells turn malignant in mammary epithelial tissues, the tissues frequently involved in breast cancer.
Dense mammary tissue has long been recognized as a strong indicator of risk for breast cancer. This is why regular breast examinations are considered essential to early detection. Until now, however, the significance of that tissue density has been poorly understood.
By isolating mechanical and biological variables one by one in vitro, Mooney and his research team have discovered how the physical forces and chemical environment in those dense tissues can drive cells into a dangerously invasive, proliferating mode. The findings were published online June 15 in Nature Materials.
"While genetic mutations are at the root of cancer, a number of studies over the last 10 to 20 years have implicated the cellular microenvironment as playing a key role in promoting or suppressing tumor progression," says lead author Ovijit Chaudhuri, a former postdoctoral fellow in the Mooney Lab at Harvard who recently joined the mechanical engineering faculty at Stanford University. The new research finds that the stiffness of the extracellular matrix and the availability of certain ligands (molecules that bind to cell membranes) can together determine which genes are actually called on—and whether normal epithelial cells begin to exhibit the behaviors characteristic of highly malignant cancer cells. "Our findings suggest that evaluating the composition of this microenvironment, in addition to mammographic density, could potentially provide a better assessment of breast cancer risk," Chaudhuri says.
Research in the Mooney Laboratory explores how the physical properties of natural biomaterials and synthetic polymers can affect how cells sense their environment, react to it, and signal to one another. The extracellular matrix—the complex network of crosslinked proteins and polymers that connects living cells to one another and facilitates communication between them—offers a challenging subject for study. The usual two-dimensional culture of cells on petri dishes has proven to be a poor model of three-dimensional tissues.
"As bioengineers, we can now design 3D culture systems where environmental parameters such as composition, porosity, and stiffness can be precisely tuned to study the importance of these cues on tumorigenesis," says coauthor Sandeep Koshy, a Harvard graduate student in the Harvard-MIT Program in Health Sciences and Technology, who works in Mooney's lab at Harvard SEAS and at the Wyss Institute for Biologically Inspired Engineering. "We're seeing that some of these factors have a major impact on cell behavior that is not possible to observe in conventional 2D cell cultures."
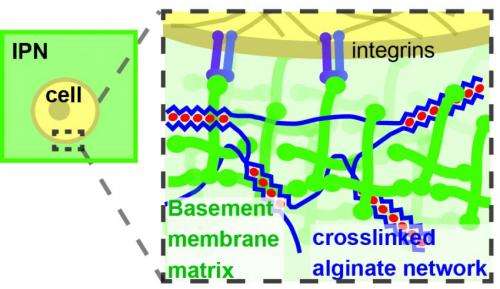
Prior studies have used varying amounts of a fibrous protein called collagen to adjust the stiffness of the extracellular matrix, but Mooney's team recognized early on that collagen has more than a simple mechanical effect on cells: it can also trigger certain signaling pathways. Fibrous collagen is not normally found in the basement membrane that surrounds the mammary epithelium, so any collagen signaling could confound the conclusions of those studies.
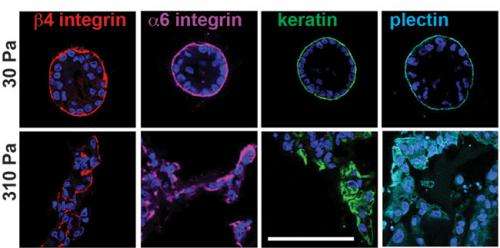
To eliminate uncontrolled variables, the team designed a new material model that uses alginate gel, instead of collagen, to stiffen the extracellular matrix without binding to any cell receptors. When this model was in its softest mode, normal (benign) mammary epithelial cells behaved normally within it, forming cellular structures referred to as acini that capture many key features of the normal in vivo mammary epithelium. But when the gel was stiffer, the cells began to upregulate the expression of cancer-related genes, and activity of the PI3K pathway that is known to drive cell proliferation and invasion increased.
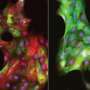
"The invasive structures we observed in our stiff matrices resemble the morphology of early-stage invasive ductal carcinoma. They also show increased expression of the estrogen receptor alpha [ER+] gene that drives cell division," says Koshy. "It is striking that these changes, found in many human cancers, can be induced in normal mammary epithelial cells simply by varying the stiffness or composition of the matrix surrounding them."
But while stiffness is a key part of the story, it is not the full story. Further experiments indicated that the cells would recover their normal behavior in high-stiffness gels if they were exposed to increasing concentrations of laminin, a protein naturally found in the basement membrane.
When the extracellular matrix is very flexible, or when a high concentration of laminin is readily available, proteins called α6β4 integrins within the cell membrane bind with laminin to form small structures called hemidesmosomes, which anchor the epithelial cells to the basement membrane. But fluorescence microscopy revealed that the cells in a stiff matrix were not forming hemidesmosomes at all, so Mooney's team hypothesized that a stiffer matrix and a shortage of laminin was leaving the α6β4 integrins with dangling, unbound tails.
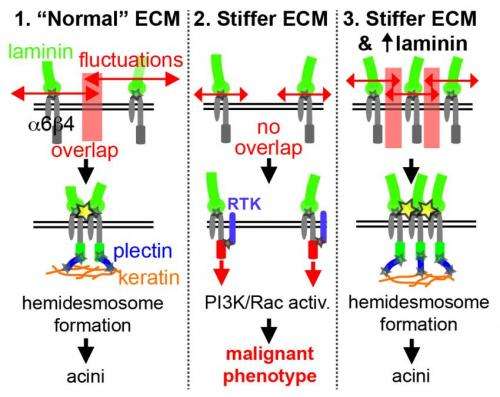
The team's final experiments demonstrated that these unbound integrin tails, indeed, get up to no good: they participate in the activation of two key biochemical pathways (PI3K and Rac1) that are necessary and sufficient to induce malignant cell behaviors in the in vitro epithelial tissue.
"If further studies validate that these processes are critical in human breast cancers," Koshy notes, "the possibility exists that agents that favorably modify the biophysical properties of the extracellular matrix, or that target the receptors and signaling molecules associated with how cells sense this matrix, could be used as a new avenue for the prevention or treatment of breast cancers."
In addition to the implications of this research for cancer biology, the development of the alginate-based extracellular matrix model is also significant.
"Studies of many other biological processes could benefit from the use of this system," says Mooney, who in addition to his role at Harvard SEAS is also a Core Faculty Member at the Wyss Institute. "Studies on stem cell biology, wound healing, and development in a variety of tissues and organs could utilize this system."
More information: Paper: www.nature.com/nmat/journal/va … nt/abs/nmat4009.html