Scientists hit 'delete': Removing regions of shape-shifting protein explains how blood clots
In results recently published in Proceedings of the National Academy of Sciences (PNAS), Saint Louis University scientists have discovered that removal of disordered sections of a protein's structure reveals the molecular mechanism of a key reaction that initiates blood clotting.
Enrico Di Cera, M.D., chair of the Edward A. Doisy department of biochemistry and molecular biology at Saint Louis University, studies thrombin, a key vitamin K-dependent blood-clotting protein, and its inactive precursor prothrombin (or coagulation factor II).
"Prothrombin is essential for life and is the most important clotting factor," Di Cera said. "We are proud to report that our lab here at SLU has finally succeeded in crystallizing prothrombin for the first time."
Blood-clotting has long ensured our survival, stopping blood loss after an injury. However, when triggered in the wrong circumstances, clotting can lead to debilitating or fatal conditions such as a heart attack, stroke or deep vein thrombosis.
Before thrombin becomes active, it circulates throughout the blood in the inactive (zymogen) form called prothrombin. When the active enzyme is needed (after a vascular injury, for example), the coagulation cascade is initiated and prothrombin is converted into the active enzyme thrombin that causes blood to clot.
X-ray crystallography is one tool in scientists' toolbox for understanding processes at the molecular level. It offers a way to obtain a "snap shot" of a protein's structure.
In this technique, scientists grow crystals of the protein they want to study, shoot x-rays at them and record data about the way the rays are scattered by crystals. Then they use computer programs to create an image of the protein based on that data.
Once scientists can visualize the three dimensional structure of a molecule, they can begin to piece together the way in which the protein functions and interacts with other molecules in the body, or with drugs.
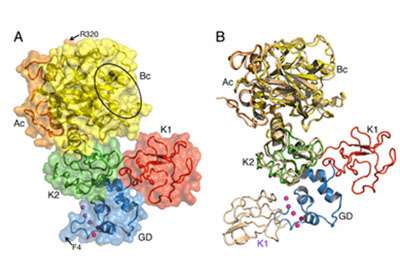
Last year, Di Cera and colleagues published the first structure of prothrombin. This first structure lacked a domain responsible for interaction with membranes and certain other sections were not detected by x-ray analysis. Though the scientists were able to crystallize the protein, there were disordered regions in the structure that they could not see.
Within prothrombin there are two kringle domains (looped sections of a protein named after the Scandinavian pastry) connected by a "linker" region that intrigued the SLU investigators because of its intrinsic disorder.
"We deleted this linker and crystals grew in a few days instead of months, revealing for the first time the full architecture of prothrombin," Di Cera said.
In addition to this remarkable discovery, Di Cera and colleagues found that the deleted version of prothrombin is activated to thrombin much faster than the intact prothrombin. The structure without the disordered linker is in fact optimized for conversion to thrombin and reveals key information on the mechanism of prothrombin activation.
For over four decades, scientists have tried to crystallize prothrombin but without success.
"It took us almost two years to discover that the disordered linker was the key," Di Cera said. "Finally, prothrombin revealed its secrets and with that the molecular mechanism of a key reaction of blood clotting finally becomes amenable to rational drug design for therapeutic intervention."
More information: Nicola Pozzi, Zhiwei Chen, Leslie A. Pelc, Daniel B. Shropshire, and Enrico Di Cera, The linker connecting the two kringles plays a key role in prothrombin activation, PNAS 2014 ; published ahead of print May 12, 2014, www.pnas.org/content/early/201 … /1403779111.abstract
















