Structure of enzyme seen as target for ALS drugs
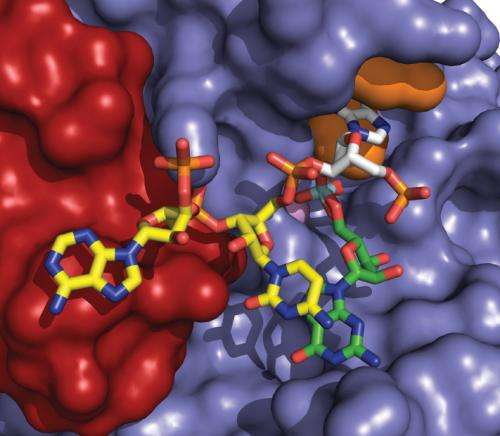
Investigators from the School of Medicine at The University of Texas Health Science Center at San Antonio have determined the first high-resolution structure of an enzyme that, if partially inhibited, could represent a new way to treat most cases of amyotrophic lateral sclerosis (ALS, also called Lou Gehrig's disease).
Dbr1 is the only enzyme known to break loops of ribonucleic acids (RNAs) called intron lariats. When Dbr1 activity is high, very few of these lariats remain in cells.
"This is relevant to ALS because another protein called TDP-43 aggregates to form clumps in motor neurons in 50 percent to 80 percent of all ALS cases," said senior author P. John Hart, Ph.D., professor of biochemistry and director of the X-ray Crystallography Core Laboratory at the UT Health Science Center San Antonio. "Decreasing Dbr1 activity will cause some of these lariats to remain and when TDP-43 binds these lariats, it is prevented from forming clumps in motor neurons."
ALS is characterized by death of motor neurons, resulting in progressive paralysis.
"This is the first picture of this enzyme to be obtained," Dr. Hart said. "We can see all the details, which will help us to develop small molecules (drugs) to inhibit Dbr1 activity."
The other lead investigators on the paper, published as a "Breakthrough Manuscript" in Nucleic Acids Research, are Masad Dahma, Ph.D., professor and chair of the Department of Chemistry at McGill University in Montreal, and Scott Stevens, Ph.D., associate professor of molecular genetics and microbiology at The University of Texas at Austin.