Center announces development of experimental treatment for myelodysplastic syndromes
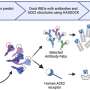
In a major step to treat patients living with Myelodysplastic Syndromes (MDS), a group of diseases that affect the bone marrow and blood, Moffitt Cancer Center today announced the development of an innovative investigational biologic agent that could improve patient response and outcomes for MDS and other diseases.
Working toward making the treatment available to patients, Moffitt and Celgene Corporation (Nasdaq:CELG) have entered into a licensing agreement that gives Celgene the exclusive rights to the novel, investigational MDS therapy. The agents, developed by Alan F. List, M.D., Moffitt CEO and president, and Sheng Wei, M.D., senior member of Moffitt's Immunology Program, also has potential applications for autoimmune diseases, inflammation and enhancement of anti-cancer immune responses.
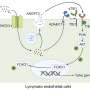
The researchers at Moffitt discovered a strong linkage between inflammatory pathways, tumor microenvironment, innate immunity and the development of MDS. This led to the development of the licensed technologies, which could potentially improve treatments for MDS patients.
"Currently there are limited therapeutic options for patients and few programs targeting the underlying causes of this disease. This agent is a first-in-class, novel strategy for targeting a key soluble effector of the disease that could be transformational in our understanding and care of MDS," List said.
More than 50,000 people are diagnosed with MDS in the United States each year. Although MDS can affect people of any age, the majority are older than 60. The cause of MDS is unknown, but exposure to certain industrial chemicals or radiation can increase the risk of developing the disease. Few approved options are available for patients with this rare disease.
"Our agreement with Moffitt emphasizes Celgene's commitment to identify and develop innovative therapies that advance the treatment of cancer and other diseases, particularly scientific approaches that meaningfully target fundamental disease mechanisms," said Rajesh Chopra, M.D., corporate vice president, Translational Development at Celgene. "We are very enthusiastic about Moffitt's leading disease expertise in MDS and the possibilities for this license agreement and other potential scientific programs."
"Celgene has a tradition of highly effective collaborations with organizations that have a deep scientific approach to research. We believe that by partnering Moffitt's commitment to cutting-edge research with Celgene, a long-established leader in hematology and MDS, this novel technology can be translated into a meaningful clinical option for patients with MDS," said Wei.
In pursuing new and innovative cancer prevention and treatment methods, Moffitt's research attracts high-paying, high-skills jobs and millions in federal grant funding to the area. Research plays an important part in Moffitt's overall impact on the community, which includes more than 4,500 jobs in Florida and more than $1.6 billion in direct economic impact.
Under the agreement, Celgene has the option to partner on five additional scientific programs, which Moffitt may elect to pursue, including immunotherapy and cell signaling. Financial considerations were not disclosed.
List is a member of Celgene's Scientific Advisory Board.