Study reveals key differences in how primary tumors, metastasis respond to neoadjuvant antiangiogenic therapy
In a joint effort with the Sunnybrook Research Institute (SRI) in Toronto, Roswell Park Cancer Institute (RPCI) researchers have developed a novel preclinical methodology for examining the effects of neoadjuvant therapy in animal models. Their approach may enable oncologists to distinguish which antiangiogenic therapies, or treatments designed to block blood vessels from assisting tumor growth, are optimal for improving overall survival after treatment has stopped and the tumor has been surgically removed.
"Typically, antiangiogenic therapy is used in patients with cancer that has spread, or metastasized, throughout the body, often after a primary tumor has been surgically removed," said John M. L. Ebos, PhD, an Assistant Professor of Oncology in the Genitourinary Section of the Department of Medicine at RPCI. "Clinical trials are now testing whether giving antiangiogenic therapy before surgery may have some benefit, both to improve the surgery itself—because a smaller tumor is easier to remove—and to prevent or delay the disease from coming back. But because these presurgical treatments, termed 'neoadjuvant' therapy, have not been tested in the preclinical setting, these benefits have not been confirmed."
Dr. Ebos and his colleagues conducted the research to compare various neoadjuvant antiangiogenic treatments and evaluate their impact on metastasis and survival. According to Dr. Ebos, neoadjuvant therapies are rarely tested in animal models because surgically removing tumors is difficult and most tumor cell lines are not prone to metastasis. The laboratory of Robert S. Kerbel, PhD, a collaborator and former mentor to Dr. Ebos from the SRI, which is affiliated with the University of Toronto, has developed unique animal tumor models with cells that efficiently metastasize after surgery and allow for studying presurgical treatments.
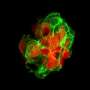
The researchers studied spontaneous metastatic disease in animal models by implanting the kidney, breast, and melanoma cells into their respective organs and then surgically removing the primary tumor. They then identified the optimal surgical parameters necessary for comparing the effects of short-term neoadjuvant treatment on postsurgical metastatic recurrence after stopping therapy. "Our results show that treatment in the presurgical setting is generally good—that is, that it slows primary tumor growth—but this does not always mean survival will be improved once therapy is stopped and the tumor is removed," Dr. Ebos says.
Dr. Ebos and colleagues evaluated different types of currently approved antiangiogenic drugs that block the vascular endothelial growth factor receptor (VEGF) pathway: small-molecule receptor tyrosine kinase inhibitors (RTKIs) that block the receptor from being activated and antibodies that may block the function of the VEGF. The study results showed that antibodies—for example, animal equivalents of the clinically approved VEGF antibody bevacizumab—slowed tumor growth before surgery and improved survival after neoadjuvant therapy.
"However, the results with RTKIs are more variable," Dr. Ebos says. "Under certain conditions, neoadjuvant benefits before surgery led to reduced or even worsened effects after surgery. This seems dependent on how the drug is used." Negative postsurgical effects could be improved with increased dose, shorter treatment duration and earlier surgical times, according to Dr. Ebos. "Our results show that all of these are critical considerations when testing this therapy, and all have an impact on the results and how they are interpreted."
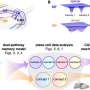
Based on these data, the researchers have established a scoring system for determining drug combinations that will maximize neoadjuvant treatment benefits. "By comparing the effects of neoadjuvant treatment in our unique models, we have the opportunity to distinguish where a drug may work best and how best to use them," Dr. Ebos says. "Our study provides examples of potential drug combinations, such as antiangiogenic therapy with chemotherapy given in low doses, as a proof-of-principle to improve the overall benefits."
More information: Ebos, J. M. L., Mastri, M., Lee, C. R., Tracz, A., Hudson, J. M., Attwood, K., Cruz-Munoz, W. R., Jedeszko, C., Burns, P. and Kerbel, R. S. (2014), "Neoadjuvant antiangiogenic therapy reveals contrasts in primary and metastatic tumor efficacy." EMBO Mol Med. doi: 10.15252/emmm.201403989