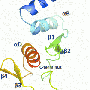Rift Valley fever virus' proteins imitate human DNA repair factors
A potential mechanism to combat diseases caused by haemorrhagic fever viruses has been discovered by researchers at the University of Montreal's Department of Biochemistry and Molecular Medicine. These diseases present a dramatic risk to human health as they often spread quickly and kill a high percentage of infected individuals, as demonstrated by the recent Ebola outbreaks. Effective treatments such as vaccines and drug therapies are not available for many of these infections since the outbreaks mostly occur in developing countries with limited financial resources. Moreover, the genomes of many haemorrhagic fever viruses mutate rapidly, enabling them to quickly adapt to potential drug treatments and evade the immune system.
"Although our work does not directly lead to treatments on a short term, it does identify a process where the virus could be vulnerable to drug therapy, or how we might design an attenuated viral strain for vaccine development," said first author Normand Cyr, a postdoctoral researcher. "Identification of the Achilles heels of haemorrhagic fever viruses like the Rift Valley fever virus will help inspire additional research and eventually lead to the development of new therapeutic strategies to treat these deadly tropical infections."
The research was supervised by senior co-author Professor James Omichinski and published in the Proceedings of the National Academy of Sciences (PNAS). "Our group used Nuclear Magnetic Resonance (NMR) spectroscopy studies to investigate the structural properties of an important viral protein required for virulence of the Rift Valley fever virus, a virus that causes infections in both humans and livestock similar to the Ebola virus," Omichinski explained. "Viral proteins such as the Non-structural protein (NSs) studied here bind to the transcription machinery of human cells via the p62 subunit of the TFIIH protein complex, which leads to the formation of nuclear filaments that are essential for propagation of the virus. The structural details reported show that the viral protein uses a simple so-called ΩXaV motif that is similar to that found in human DNA repair proteins, and blocking this binding event with drugs would certainly weaken the virulence of the virus."
"Viruses and other infectious agents mutate and constantly adapt to treatments. Therefore, it is critical to conduct this type of basic research so that humans can stay one step ahead of potential outbreaks of viral infections, which is one of the core missions of our Department," said Professor Christian Baron, Chair of the Department of Biochemistry and Molecular Medicine. "The structural biology facilities at Université de Montréal are cutting edge, thanks to important investments from the Canada Foundation for Innovation, and these facilities are helping us to unravel the molecular details of how the Rift Vally fever virus functions," Omichinski added. The University of Montreal team worked in collaboration with senior co-author Kylene Kehn-Hall's group at the National Center for Biodefense and Infectious Diseases in the United States, as the US team has specialized biosafety level 3 facilities where they can work with such contagious viruses.
Indeed, Americans and Canadians have every reason to be concerned about the future of this line of research. "Climate changes and world-wide travel are increasing the risk of haemorrhagic fever viruses even in Canada. Warmer temperatures and increased travel are bringing such tropical diseases much closer to home and as a result we cannot afford to ignore the global health status of populations in other countries. It is therefore critical that we gain more knowledge into the molecular details of viral function so that we can develop more effective treatments and control the spread of these diseases," Omichinski said.
More information: A ΩXaV motif in the Rift Valley fever virus NSs protein is essential for degrading p62, forming nuclear filaments and virulence, DOI: 10.1073/pnas.1503688112