Considerable symptom burden with adjuvant endocrine Tx

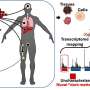
(HealthDay)—Patients treated with adjuvant endocrine therapy (ET) for primary breast cancer have considerable symptom burden, according to a study published online Jan. 19 in the Journal of Clinical Oncology.
In a prospective, observational cohort study, Patricia A. Ganz, M.D., from the University of California, Los Angeles, and colleagues examined patterns of health and symptoms associated with the initiation of adjuvant ET for primary breast cancer treatment at enrollment and six and 12 months later. Two-thirds of the 186 women enrolled initiated ET (evenly split between aromatase inhibitor [AI] and tamoxifen).
The researchers found that initial physical health scores were below normative levels and improved over time; at 12 months, the score was significantly lower in the AI group (P = 0.05). Mental health scores were within normal range, with no between-group difference or change over time. Symptom severity was either stable or declining in the non-ET group, while the ET groups frequently had increased symptom severity over time. Compared with the no-ET group, the AI group reported more severe musculoskeletal, hot flash, and cognitive problems at one or both follow-up time points, while the tamoxifen group had higher levels of hot flashes, cognitive problems, and bladder problems.
"Attention should be given to the increased symptom burden associated with ET, and better efforts should be made to address patient-reported outcomes," the authors write.
One author disclosed financial ties to the biopharmaceutical industry.
More information:
Abstract
Full Text (subscription or payment may be required)
Copyright © 2016 HealthDay. All rights reserved.