January 22, 2016 report
Magnesium-based orthopedic implant that safely biodegrades and promotes bone growth
(Medical Xpress)—Researchers from South Korea have shown, in preliminary clinical trials of 53 patients, that after a year their magnesium-calcium-zinc orthopedic implant spurs new bone growth by mimicking the body's calcification matrix while the implant safely degrades. Their work appears in the Proceedings of the National Academy of Sciences.
Orthopedic implants in the form of screws, rods, or metal plates are used to hold fractured bones in place so that they heal properly. Oftentimes this involves an initial surgery to insert the device followed by a second surgery to remove it. An alternative to this would be a biodegradable implant. Researchers have explored this idea using magnesium-based implants since magnesium is known to degrade within the body. However, there has been limited success in this area.
Lee, et al. sought to understanding how magnesium-alloy implants interact with the surrounding bone on the molecular level in an effort to produce alloys that biodegrade and spur new bone growth. Prior work by them had shown that Mg-5wt%Ca-1wt%Zn can serve as a strong implant that will degrade over time. Additionally, the calcium and zinc components are sufficient to promote bone formation and remodeling, respectively. In this paper they report the results of a long-term clinical study in which 53 patients with a fracture in their hand were given a Mg-5wt%Ca-1wt%Zn bone implant, and the surrounding bone was studied at the macro-, micro-, and nano-levels to determine what is happening in the bone at these levels.
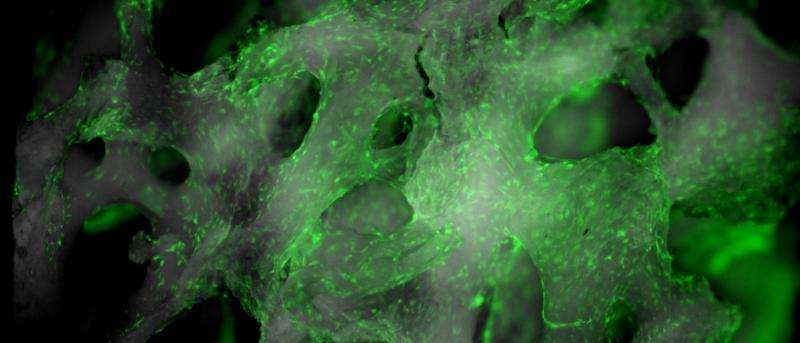
Using histological staining techniques, they were able to distinguish between new and old bone at the site of the implant. They investigated new bone sites using SEM and TEM and found that the new bone formation sites seem to be a result of the implant's degradation products, which induced the body's natural bone forming system.
The area of study was further investigated by dividing the bone into several regions radiating from the magnesium alloy implant and fracture interface. Region I is located at the interface with subsequent regions (i.e., Regions II, III, IV, etc.) extending outward away from the interface. The different regions were determined based on fluorescence and SEM studies indicating varying chemical composition.
They found that Region I had high magnesium and oxygen content, Region II had high calcium and phosphorous content, and Region III displayed a typical bone-like microstructure. Within Region II, there were two types of morphologies, an amorphous ring pattern and crystalline structures. The authors believe that Region I is predominantly Mg(OH)2 while Region II has both calcium phosphate (Ca3(PO4)2) and hydroxyapatite (Ca5(PO4)3OH).
Magnesium hydroxide is a corrosion product, and indeed, studies showed that the magnesium alloy implant progressively degraded throughout the clinical trial up to a year out when the implant was barely distinguishable. As degradation occurred, new bone grew in its place. The chemical composition closest to the implant-bone interface showed Mg(OH)2, which set off other reactions due to changes in pH levels. These changes lead to the formation of calcium phosphate compounds with the part of Region II closest to Region I displaying amorphous calcium compounds, and the part of Region II farther way displaying crystalline hydroxyapatite.
This crystalline product mimics the body's natural calcification matrix causing it to get resorbed by the osteoclasts and induce bone formation by the osteoblasts. This continuous process of magnesium alloy degradation and bone formation allows the implant to eventually disappear and new bone to appear.
Notably, after the one-year follow up with the 53 patients, all of them healed within the expected timeframe and none of them experienced pain from implant degradation. Further studies to determine degradation rate are needed to further predict how the Mg-Ca-Zn alloy will work in the body, as well as additional studies at other bone fracture sites. This research provides a promising new avenue for orthopedic implant design that not only seems to safely biodegrade but also promotes new bone growth in the process.
More information: Jee-Wook Lee et al. Long-term clinical study and multiscale analysis of in vivo biodegradation mechanism of Mg alloy, Proceedings of the National Academy of Sciences (2016). DOI: 10.1073/pnas.1518238113
Abstract
There has been a tremendous amount of research in the past decade to optimize the mechanical properties and degradation behavior of the biodegradable Mg alloy for orthopedic implant. Despite the feasibility of degrading implant, the lack of fundamental understanding about biocompatibility and underlying bone formation mechanism is currently limiting the use in clinical applications. Herein, we report the result of long-term clinical study and systematic investigation of bone formation mechanism of the biodegradable Mg-5wt%Ca-1wt%Zn alloy implant through simultaneous observation of changes in element composition and crystallinity within degrading interface at hierarchical levels. Controlled degradation of Mg-5wt%Ca-1wt%Zn alloy results in the formation of biomimicking calcification matrix at the degrading interface to initiate the bone formation process. This process facilitates early bone healing and allows the complete replacement of biodegradable Mg implant by the new bone within 1 y of implantation, as demonstrated in 53 cases of successful long-term clinical study.
© 2016 Medical Xpress