Engineering a solution to cancer

I was stunned when I entered the hospice room. A shriveled, elderly woman sat in the corner, her tiny frame emphasized by the oversize chair that held her. It was hard to believe this was the irrepressible lady I had seen four months previously – she didn't look like my grandmother. Amongst many things, my grandmother loved watching birds, and if they came to feed in her garden she would be up reaching for the binoculars. Now, ignoring the twittering outside, she sat facing inwards under a blanket – only half alive.
Watching cancer lay waste to someone I loved was the worst thing I have ever experienced; I felt totally helpless, and there was absolutely nothing that could be done. Last week saw reports of >90% remission rate in a clinical trial of a new treatment given to patients suffering from terminal Acute Lymphoblastic Leukaemia (ALL). To someone like me who has no training in oncology, it sounds like a modern miracle. But we have been here before and people are still suffering, so can we expect it will be different this time?
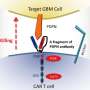
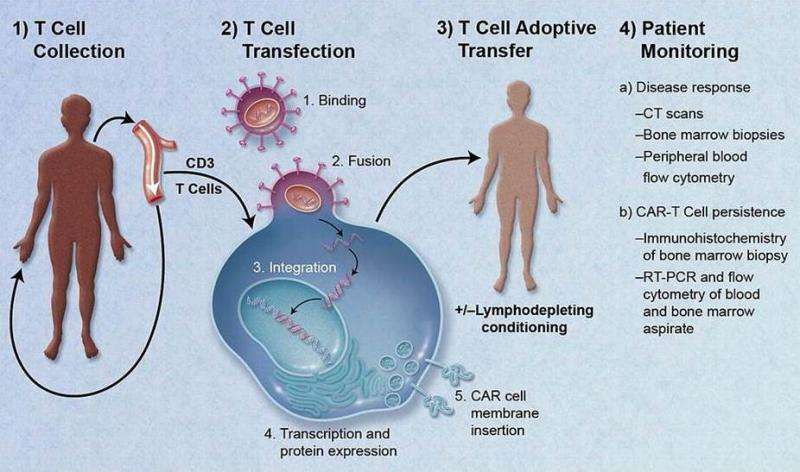
The treatment in question is called Chimeric Antigen Receptor T-cell therapy (CAR-T). It is based on the idea of engineering T-cells to recognize and destroy tumors, and is at the forefront of synthetic immunotherapy. However, CAR-T is not actually new; the first report of synthetic T-cells was published in 1989 by Prof. Zelig Eshhar and colleagues, so I was curious why are we only hearing about it now. To assess the importance of the latest announcement I contacted Dr Filippo Menolascina of SynthSyS at the University of Edinburgh for a Q&A, as he highlighted advances in CAR-T as a major development during the PLOS Synbio review of 2015.
SJB: Can you tell us what makes CAR-T special?
FM: CAR-T offers an opportunity we have rarely had before: homing in on the genetic basis of cancer cells to identify them and effectively kill them, one by one. Selectivity is what sets CAR-T apart from the rest of the cancer therapies used in clinical practice: surgery, radiotherapy and chemotherapy all largely fail in precisely discriminating between "good" and "bad" cells, leaving behind them the trail of infamous side effects the fight against cancer is riddled with. The principle behind CAR-T is simple yet very powerful: if we know the molecular signature of cancer cells we can "teach" our immune system how to get rid of them.
SJB: What is you view of the recent announcement?
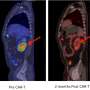
FM: 24 out of 27 patients presenting refractive acute lymphoblastic leukemia (ALL), a treatment-resistant form of cancer that prevents the bone marrow from making the normal constituents of blood, were brought into complete remission using CAR-T. An "unprecedented result" some claimed. And indeed it is a major achievement: although most ALL patients (from 67.5% to 90%) will respond to conventional therapies (chemotherapy/stem cell transplant) and survive, before CAR-T, a large fraction of the few that did not respond to therapies had to face a terrifying prognosis: rapid progress of the disease and death. Now these patients, however small of a cohort (ALL killed 1450 in 2015 in the US), have one more, concrete and powerful therapeutic option. Yet it is crucial to clarify some aspects of CAR-T and avoid the generation of unreasonable hype, or worse, unrealistic expectations in cancer patients.
SJB: In what way?
FM: Claiming that "scientists have finally found the way to cure cancer", as some press dubbed the recent news, is brutally misleading. Although it builds upon a therapy originally developed for solid tumors, the efficacy of CAR-T has largely been demonstrated up to now on non-solid cancers, i.e. blood cancers. The so called "big killers" (lung, colon, breast and prostate cancer, ~275000 deaths in the US in 2015), on the other hand, all belong to the class of solid-cancers. Although several companies have announced CAR-T-based clinical trials for these tumors the efficacy of such approaches remains to be established. Of concern, in particular, is the effect of the microenvironment that cancerous masses build around them: unlike blood cancers, solid tumors secrete potent chemical signals that trick CAR-T cells into thinking that they should not really be spending time in those areas. Will we be able to teach CAR-T cells a new lesson: how to not to be tricked by tumors? Although some strategies have been proposed (see "Armored T-Cell" proposed by Juno) this remains an open question.
SJB: I just wanted to pick up on the idea that CAR-T cells can be 'taught' to recognize cancer – how does this actually work?
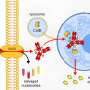
FM: CAR-T only works "if" a molecular signature of cancer is known. So far the only types of cancers we found a robust identifier for are ALL, non-Hodgkin lymphomas and Chronic Lymphocytic Leukemia (cancer cells of these types all present a specific molecule, antigen CD19, on their surfaces, making them "more easily" identifiable). What if such a simple, "CD19: yes/no?", signature is not available? Then we have to find other, potentially more complex, signatures. For example: both CD19 and CD22 have been found to be characteristic of some types of cancer of the immune system. How can we program CAR-T cells to encode a rule of the type: IF "CD19 is PRESENT" and "CD22 is PRESENT" THEN "KILL"? This is what synthetic biologists are looking into. As a matter of fact, such rules are essentially "elementary computer programs", classifiers in this specific case, of the type we routinely encode in the genome of synthetic cells to "program" them to perform new functions. Engineering "intelligence" in CAR-T cells is one of the most exciting perspectives in this field and a unique opportunity to unlock the full potential of this approach.
SJB: Are there other challenges that need to be addressed before this treatment is widely used?
FM: We need to shed light on the most prominent side-effect of CAR-T, Cytokine Release Syndrome (CRS) or "Cytokine Storm". In roughly a third of the patients, the newly injected CAR-T cells generated such a strong inflammatory response that a spectrum of symptoms ranging from hypotension, to high fevers and pulmonary edema was observed. Complications of this type led to half-dozen treatment-related deaths in clinical trials. While most of the fatalities were observed in patients with pre-existing medical conditions, synthetic circuits are being developed as countermeasures (e.g. drug-inducible "kill-switches" that stop T-cells when needed). Finally, mitigating the effect of CRS will be key to attack solid tumors, the real chance for CAR-T to become a game changer: there the chances to attack healthy cells will be much higher than in the blood stream and the perspective of ending up in a life-threatening situation worryingly dangerous. Engineering more "intelligence" or "control" in the CAR-T cells might be a solution to this problem; time will tell whether it will work.
There is no such thing as a miracle; my grandmother died from an inoperable brain tumor four years ago, and for many like her effective treatments remain a distant possibility. However, as the development of CAR-T for ALL patients demonstrates, armed with knowledge and perseverance, we can continue to take steps towards achieving what once seemed impossible.
More information: ash.confex.com/ash/2014/webprogram/Paper75620.html
seer.cancer.gov/statfacts/html/alyl.html
This story is republished courtesy of PLOS Blogs: blogs.plos.org.