Regulation of semiochemicals in inflammation
Tumour necrosis factor-alpha (TNF-α) is a central signalling substance of the immune system and involved in many inflammatory processes. Blocking this molecule is the foundation of modern treatment against inflammatory diseases such as rheumatism, psoriasis or chronic inflammatory bowel diseases. The molecular mechanism on which the release and therefore activation of TNF-α is based was first clarified by a working group from the Cluster of Excellence "Inflammation at Interfaces" at the Faculty of Medicine at Kiel University. The team, led by cell biologist Professor Karina Reiß, therefore achieved a pioneering success. This is because the principle discovered is of fundamental significance and opens up a completely new area of research in cell biology. Apart from this, the study which was recently published in Nature Communications identifies new starting points for the development of anti-inflammatory treatments.
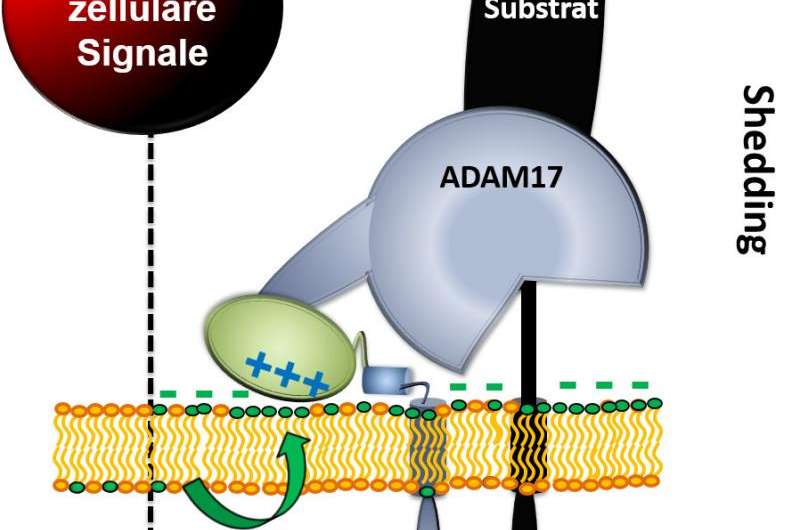
Since the central role of tumour necrosis factor-alpha (TNF-α) has become known in the inflammatory process, working groups throughout the world have been trying to understand how this important molecule is regulated in the body. A milestone in this connection was a discovery made in 1997. At that time the enzyme was discovered which releases TNF-α. The enzyme, called ADAM17, sits on the cell surface and "cuts off" TNF-α there from the primary stage embedded in the membrane, so that it can unfold its effect on other cells. In addition to TNF-α, ADAM17 also splits a large number of other molecules located in the membrane and therefore conveys them in a soluble form. "The protease cuts off substrates directly via the cell membrane. Then these proteins that have now been released can bind themselves to receptors on other cells. This is how the protease ADAM17 regulates an unbelievable amount in our bodies", explains Karina Reiß, who has been carrying out research as a professor for epithelial protease inhibitors in the Cluster of Excellence "Inflammation at Interfaces" and the Department of Dermatology, Venerology and Allergology at Kiel University's Faculty of Medicine and the University Medical Center Schleswig-Holstein (UKSH) since 2008. A large number of substrates have already been identified that ADAM17 splits. These play a role in such things as cell proliferation, i.e. the growth of tissue, and in immune reactions.
Since the discovery of ADAM17 as a TNF-α releasing enzyme, it is hoped that the further characterisation of this protease will lead to progress in the treatment of people with chronic inflammatory bowel diseases (Crohn's disease, colitis ulcerosa), skin diseases (psoriasis) or inflammatory joints (rheumatoid arthritis). The fundamental questions are: when does this enzyme start to cut off something from the cell surface, and how exactly does this work? Research has now been carried out on the substances through which the protease is activated, in other words, when it starts to cut something off. "But no one has yet understood how this works at the molecular level. We have made an important contribution to this. For the first time we can describe the mechanism of how ADAM17 is activated and therefore make a major contribution to understanding the regulation of this important enzyme", explains Reiß.
A component of the cell membrane, the lipid molecule phosphatidylserine, abbreviated to PS, plays a key role in the process. The cell membrane, which mainly consists of phospholipids, is constructed asymmetrically. Certain lipids can only be found on the inside and some others only outside of the dual layer of the membrane. The negatively charged lipid molecule PS sits on the inside of the membrane. There, positively charged proteins can dock on via electrostatic attraction, which regulates their function. "We have observed that negatively charged phosphatidylserine comes to the outside for a short time under certain conditions, so basically flips over. This creates a negative charge outside. ADAM17 has positive charges, which interact with this negative charge. This is the decisive mechanism to activate the protease so that it cuts something off ", explains Dr. Anselm Sommer, post doc in the working group and lead author of the study. It is known that the function of intracellular proteins is regulated via electrostatic attraction of PS, but this does not apply to proteins on the outside of the cell. Reiß continues: "This is basically a new fundamental principle in cell biology, which we have discovered." It is therefore entirely possible that not only the protease ADAM17, but also the function of other proteins is influenced by this outward turning of PS.
The researchers have provided evidence of the mechanism in cell culture studies with substances that are known to activate ADAM17. "We placed many known activators of ADAM17 on the cells and were able to use a PS-binding dye under the microscope to observe that the PS moves from the inside to the outside." The area of the ADAM17 molecule was also identified, which interacts with the negative charges of PS. Studies in a mouse model are planned for the next stage, which are intended to provide evidence of the newly discovered principle in a living organism, too.
More information: Anselm Sommer et al. Phosphatidylserine exposure is required for ADAM17 sheddase function, Nature Communications (2016). DOI: 10.1038/ncomms11523