Investigational new drug for Alzheimer's scheduled for first study in humans
Vanderbilt University scientists have received notification from the U.S. Food and Drug Administration (FDA) that testing in humans may proceed for an investigational new drug for Alzheimer's disease after more than 10 years of research by scientists at Vanderbilt University and Vanderbilt University Medical Center.
It is relatively uncharted territory for an academic drug discovery group to take a molecule from the laboratory setting to the clinical trials stage.
"The movement to the clinical phase of the research is the result of tireless colleagues reaching across disciplines in pursuit of the shared goal of hoping to someday improve the lives of individuals with Alzheimer's disease and possibly other brain disorders, such as schizophrenia," said Provost and Vice Chancellor for Academic Affairs Susan R. Wente, Ph.D. "This work exactly illustrates the critical role that basic science conducted in partnership with a world-class medical center can play in advancing knowledge in an attempt to fight a devastating disease."
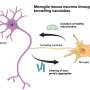
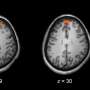
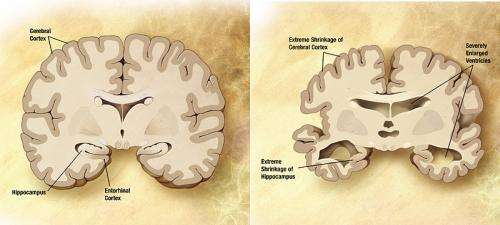
For Alzheimer's disease, the aim is for the investigational drug to target major pathologies of the disease and selectively activate a key receptor in the brain. The Vanderbilt researchers believe that the current standard of care for Alzheimer's disease, cholinesterase inhibitors, has a different mechanism of action. They are hoping to establish through future clinical testing that the molecule is broadly effective across a number of cognitive and neuropsychiatric disorders, including schizophrenia.
"This is the first instance I am aware of where an academic drug discovery group moved a molecule designed to hopefully treat a chronic brain disorder all the way from early discovery to human trials without there being, at some point along the way, a pharmaceutical partner," said P. Jeffrey Conn, Ph.D., Lee E. Limbird Professor of Pharmacology in the Vanderbilt University School of Medicine and director of the Vanderbilt Center for Neuroscience Drug Discovery (VCNDD).
"And that really is crossing what people refer to all of the time as the 'Valley of Death,' where good research discoveries have a hard time moving into the clinical testing phase due to lack of funding," he said. "Importantly, at this early stage, the FDA has only granted permission to assess potential safety of this investigational new drug in healthy volunteers" said Conn. "We cannot predict the outcome, but if these studies are successful in demonstrating that the investigational drug can be safely administered to humans, this would pave the way to allow filing of additional applications with the FDA to seek permission to advance to testing for efficacy in improving cognitive function in patients suffering from Alzheimer's disease, and possibly schizophrenia or other brain disorders. While we cannot predict the outcome of any future safety or efficacy studies, this decision by FDA allowing clinical research to begin represents a major milestone in allowing us to hopefully provide answers to those critical questions in the future."
VCNDD Co-Director Craig W. Lindsley, Ph.D., director of Medicinal Chemistry and William K. Warren, Jr. Professor of Medicine, said Phase I testing will assess drug safety and tolerability in healthy volunteer participants, a process that could take a year. If successful, the Phase II and III studies would include efficacy assessments in patients with Alzheimer's disease and could take three to five years to complete.
"We are hoping to address what we see as an unmet medical need," Lindsley said. "For Alzheimer's patients, the standard of care for symptomatic treatment remains cholinesterase inhibitors, which are 25 years old at this point. There hasn't been any real scientific advancement in this field in a long time."
Lindsley and Conn credit The William K. Warren Foundation for its philanthropic investments along the way to make clinical trials for this investigational drug a reality.
"One of the most challenging things about doing this in an academic environment is funding," Lindsley said. "Every step requires funding and if there is a delay or break in funding, then everything sits idle and potentially innovative approaches for patient care do not advance."
"Being matched with the Warrens happened serendipitously. They have invested so much in our programs, and it is wonderful to show them progress on their investments," he said. "Without the financial support from the Warrens, this investigational drug would not be poised to enter human clinical trials."
The William K. Warren Foundation Chief Executive Officer John-Kelly Warren said he is gratified that FDA has allowed for the investigational drug to proceed to testing in human beings.
"Although this is an important sequential milestone, the only milestone that matters to us is the hope that one day we will learn that this investigational new drug has positively and safely changed the life of a patient suffering from a brain disorder such as schizophrenia or Alzheimer's disease," Warren said.
"That day will warrant a celebration felt in the heavens. Until then, we are prepared to support the VCNDD research team until they can deliver the necessary results," he said.
A NIH National Cooperative Drug Discovery/Development grant funded the early basic science and discovery of this investigational drug and the Alzheimer's Drug Discovery Foundation and Harrington Discovery Institute helped support some of the key toxicity studies that FDA required, Conn said.
"The investigational new drug has the potential to improve cognitive functions with fewer unwanted side effects. This could someday be an important advance for the treatment of cognitive deficits in psychiatric disorders and Alzheimer's disease," said Joshua Gordon, M.D., Ph.D., director of the National Institute of Mental Health, which co-funded the research.