This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Scientists are closing in on a mouse model for late-onset Alzheimer's disease
Mice don't get Alzheimer's—and while that's good news for mice, it's a big problem for biomedical researchers seeking to understand the disease and test new treatments. Now, researchers at The Jackson Laboratory are working to create the first strain of mice that's genetically susceptible to late-onset Alzheimer's, with potentially transformative implications for dementia research.
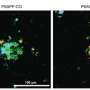
In humans, two of the defining traits of Alzheimer's disease are amyloid plaques between brain cells, and tangles of tau proteins within neurons. In mice, however, intercellular plaques don't give rise to intracellular protein tangles, so mice don't develop significant cognitive impairments.
"It's easy enough to create mouse models for studying amyloid plaques," said Greg Carter, the Bernard and Lusia Milch Endowed Chair at JAX who also leads the Model Organism Development and Evaluation for Late-onset Alzheimer's Disease (MODEL-AD) consortium with JAX colleagues Gareth Howell, Ph.D. and Mike Sasner, Ph.D. "But that replicates only a very limited aspect of Alzheimer's—and if we could cure the disease with those models, we'd have done it 20 years ago."
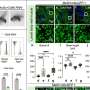
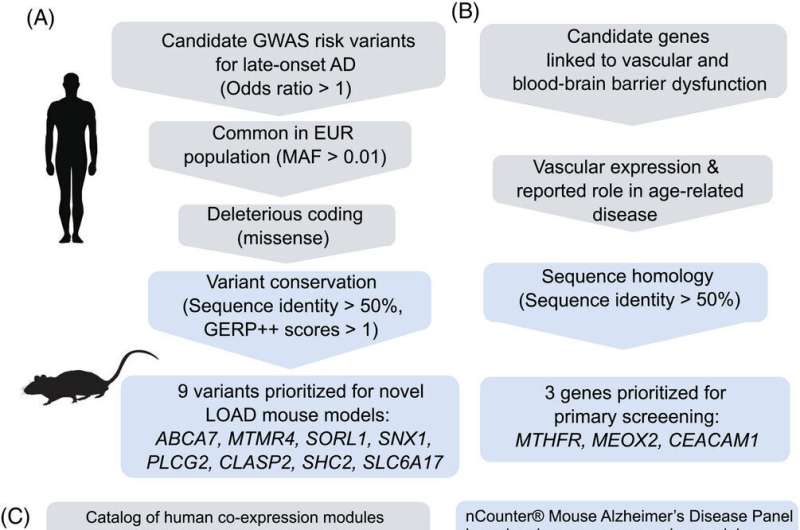
To develop new mouse strains that accurately reflect the full complexity of Alzheimer's disease, Carter's team drew on recent genome-wide studies that have identified over 70 genes associated with the illness. Using CRISPR gene editing, they collaborated with Sasner and Howell at JAX to introduce different genetic factors associated with Alzheimer's into 11 separate strains of mice, and raised the mice to maturity to explore how the genetic variants affected their brain health.
That presented a separate challenge: how can you tell when a mouse is developing Alzheimer's?
"Mice aren't very smart, so it's really hard to check whether a disease is impacting their cognitive abilities," Carter explained, whose work appears in the July issue of Alzheimer's & Dementia. "Any behavioral metric can be easily confounded by other factors like hyperactivity or sensory impairment, so focusing on mouse cognition has really led the field astray."
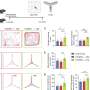
To get around that problem, the JAX team used transcriptomics—a technique that reveals the way genes are transcribed and "activated" within cells—to compare the biological signatures present in mouse brains to those of deceased human Alzheimer's patients. "By comparing the molecular biology of mouse and human brains, we can see where the two overlap, and what part of the Alzheimer's disease biology a particular gene is driving," Carter said. "It's a way to very systematically map out the disease-relevant contributions of all 11 different genetic variants."
Using transcriptomics also creates a clearer benchmark for testing new therapeutics. If a treatment aims to prevent inflammation associated with Alzheimer's, for instance, it's possible to evaluate its impact on the transcriptome signature specifically associated with inflammation.
"This approach can assess whether a treatment is working at the molecular level, without worrying so much about behavioral changes that are hard to spot in mice," said Carter. "For pre-clinical studies, it's much more precise and efficient."
First, however, Carter and team need to map the contributions of each individual genetic factor, then figure out how to combine them into a single mouse strain that shows all the key biological hallmarks of Alzheimer's. In a separate project, Carter's team tested about a dozen additional genes; now, they are working to find the most effective way to combine the genes into a single mouse model.
"We've used machine learning to figure out how to combine the genes we've studied," Carter said. "Now, we're setting up studies that will combine three or four genes at a time, and hopefully give us mice with much more complete Alzheimer's."
That's an important step forward, given that most mouse-model research into dementia focuses on the impact of single genes. "While different genes may increase your risk of dementia, no single gene causes Alzheimer's—that's not how complex genetics works," Carter explained.
"Working at JAX made it possible to raise multiple strains and study dozens of genes in parallel, and ultimately to home in on the best possible model for studying Alzheimer's Disease. We know how valuable it would be to have a mouse model for Alzheimer's disease," he added. "This research is bringing that goal within reach."
More information: Michael Sasner et al, In vivo validation of late‐onset Alzheimer's disease genetic risk factors, Alzheimer's & Dementia (2024). DOI: 10.1002/alz.13840