Knee cartilage product approved to repair defects
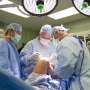
(HealthDay)—Maci (autologous cultured chondrocytes) has been approved by the U.S. Food and Drug Administration to repair defective cartilage of the knee.
The treatment, derived from healthy cartilage from the patient's own knee, uses tissue engineering to grow cells that replace the damaged cartilage, the agency said in a news release.
Cartilage defects commonly stem from an injury, knee strain, overuse, muscle weakness or general wear-and-tear, the FDA said.
The new process uses the patient's own (autologous) cells, which are placed on a collagen membrane scaffolding that is surgically implanted over the area where damaged cartilage was removed. The membrane is designed to be absorbed by the body over time.
The surgeon installing the implants should be trained in the Maci product. Multiple implants can be used if there is more than one defect, the FDA said.
Maci's safety and effectiveness were demonstrated during a two-year, 144-patient clinical study that compared the new product to an alternate surgical procedure. Potential side effects of Maci included joint pain, cold-like symptoms, headache and back pain.
Maci is produced by Vericel Corp., based in Cambridge, Mass.
More information: Visit the FDA to learn more.
Copyright © 2016 HealthDay. All rights reserved.