An elegans solution: Worm genetic screen maps cell-to-cell communication in human cancer
Some major cell-to-cell communication networks were first studied in worms. Now those worms, Caenorhabditis elegans, are being used to understand the influence of cancer mutations on those networks, report researchers at the Medical University of South Carolina (MUSC) in the May 22, 2017 issue of Developmental Cell.
Because many genes involved in cell communication are often conserved across species, C. elegans is an ideal organism to study the genes that influence them. This makes the worm a very useful genetic tool for exploring the basis of human cancer, according to Gustavo Leone, Ph.D., director of MUSC Hollings Cancer Center and the Grace E. DeWolff Endowed Chair in Medical Oncology.
"If the genetic network within tumor cells or epithelial cells is similar among C. elegans, mice and humans, the communication of neighboring cells with epithelial cells in tumors at some level might also be similar," explains Leone.
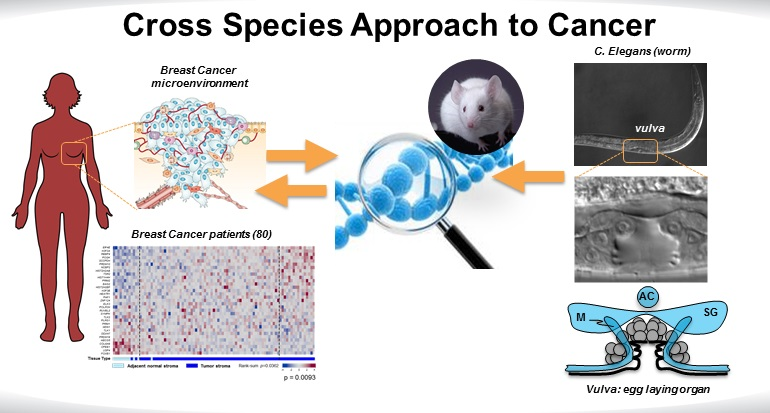
Leone is corresponding author on the study along with his colleague Helen Chamberlin, Ph.D., a C. elegans expert in The Ohio State University Department of Molecular Genetics. The two laboratories collaborated to approach a big-picture question about cancer. A number of important individual cancer genes have been discovered by Leone and many others, but is there a way to identify all of the genes-a genetic signature-involved in cell-to-cell communication in cancer? In particular, Leone sought to identify which genes within the neighboring cells that make up the tumor microenvironment could control tumor and epithelial cell proliferation.
Yet determining networks of cell-to-cell communication requires a genome-wide screen that tests genes individually, an approach that is impractical in mice.
This was where C. elegans became so essential to answering the group's question.
Part of the tumor microenvironment is supported by mesodermal cells, which send molecular signals to epithelial cells that tightly control their proliferation. This mesodermal-epithelial communication is needed in normal conditions, such as during pregnancy and wound healing, but is disrupted in cancer.
Similar communication exists between those cells in the egg-laying organ of C. elegans called the vulva. When similarly disrupted during worm development, this network can unleash epithelial cell proliferation that causes a multivulva, or Muv, feature. This feature, which becomes prominent when adult worms reach one millimeter in length, is easily visible under a microscope.
First author Huayang Liu, Ph.D., was a student in Leone's laboratory who helped design and build the genome-wide screen to identify which mesoderm genes worms need to prevent such Muv defects. Very importantly, the worms were also given a human cancer mutation in the gap-1 gene to sensitize their epithelial cells to communication signals that encourage proliferation.
In this way, the screen was designed to test the influence of each of the nearly 20,000 C. elegans genes on the proliferation of epithelial cells carrying a common cancer-sensitizing mutation.
From the entire C. elegans genome, the screen uncovered 39 worm mesoderm genes that, when reduced in expression, encouraged microscopic Muv defects suggestive of epithelial cell proliferation. Thirty-three of those genes are conserved in humans.
The identities of those genes were unexpected, according to the authors. They are not involved in 33 random processes that control cell behavior. Rather, many of them converge on hubs of regulation that control major gene expression signatures.
It appeared that the mesodermal-epithelial communication network containing this 33-gene signature could be fundamental to cell behavior in worms. Yet was it relevant in higher animals?
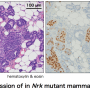
The group tested three of these 39 genes in female mice and found that reducing their expression within fibroblasts (another mesodermal-type cell) encouraged proliferation in mammary epithelial cells.
There was a final need to prove the relevance of this work to human cancer. Tests were performed in the stroma-part of the microenvironment-of tumor samples taken from human breast cancer patients. As suspected, the expression of those 33 genes was very different between normal and tumor stroma. In further experiments, depletion of 22 of these genes in human fibroblasts encouraged proliferation of breast tumor epithelial cells.
The group had confirmed a genetic signature of mesodermal-epithelial communication unique to cell proliferation in cancer.
This study uncovered a small sector of the network that allows mesodermal and epithelial cells to communicate. Yet the screen is designed to work with many cancer-sensitizing genes other than gap-1, which can reveal more of the network. Leone's group has repeated the screen using another genetic mutation that seems to influence completely different cellular processes involved in cell-to-cell communication.
A complete roadmap will guide new cancer therapies, according to Leone.
"This provides an avenue to understand why drugs work or don't work, and it provides new targets that we can now begin to drug," says Leone.
More information: Developmental Cell (2017). DOI: 10.1016/j.devcel.2017.04.024