Home bladder cancer tests set for clinical trial

A leading car sensor manufacturer is developing a device to radically simplify bladder cancer testing.
The non-invasive device uses biosensors to test urine and avoids uncomfortable follow up tests where tubes are inserted into the urethra to access the bladder.
In an industry-university collaboration led by SMR Technologies in Adelaide, researchers from the University of South Australia and Flinders Medical Centre are preparing the device for hospital trials.
The SMR Technologies device is based on research by the University of South Australia's Future Industries Institute, which identified a new polymeric compound that binds to cancer specific antibodies in urine.
These compounds are now being turned into nano-structured coatings and combined with point-of-care biosensors in a portable urine test.
SMR Technologies is one of the largest manufacturers of passenger car rearview mirrors in the world with 24 per cent of the global market share in production of exterior mirrors for light vehicles. It is also one of the leading experts for camera-based sensing systems.
However, the decline of the car manufacturing industry in South Australia in recent years has caused the company to seek opportunities in the medical device industry.
An SMR Technologies spokesman said the company was very interested in exploring opportunities in the area of cancer research.
"Cancer is a global issue and we hope that these sensors will play a key role in the fight against the deadly disease," he said.
"The funding is focused on a revolutionary diagnostic device for non-invasive early detection of urothelial cancers.
"These cancers have very high recurrence rates and ongoing patient monitoring currently requires highly invasive techniques."
The spokesman said preliminary results suggested it was a superior method to common cancer detections methods such as cytology and endoscopy.
It is now being scaled up in a AU$9.2 million project and will be trialled on 1000 patients at Flinders Medical Centre to further test its commercial potential.
According to the American Cancer Society, bladder cancer is the world's fourth most common form of the disease. About 77,000 people in the United States were diagnosed with bladder cancer in 2015.
The general five-year survival rate for people with bladder cancer is 77 per cent.
The initial diagnosis of bladder cancer usually follows symptoms such as bloody urine or frequent urination.
Patients are then required to undergo flexible cystoscopy, where a thin tube with a camera and light on the end is inserted through the urethra to the bladder.
Bladder cancer survivors are also subject to regular endoscopies because of the high probability of cancer recurrence.
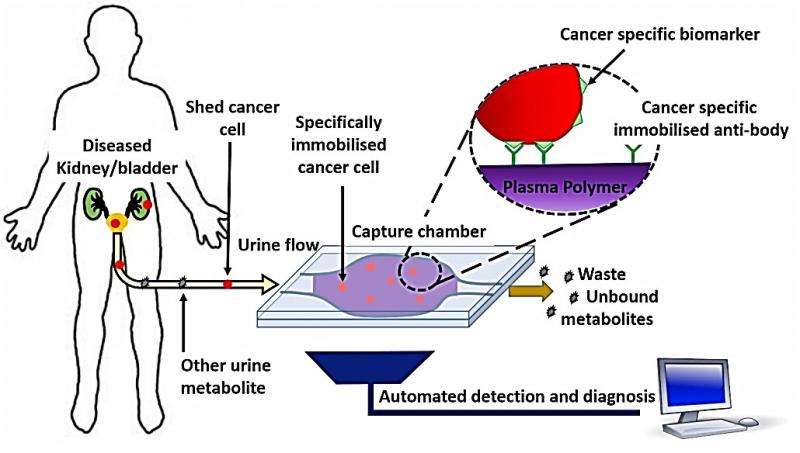
The new detection device uses the polymer coating technology to bind to the cancer cells in urine samples.
It then uses unique biosensors and micro-optics to identify the presence of those cells.
The non-invasive process enables survivors to test for cancer recurrence in the privacy of their own home without the need to undergo additional cystoscopies.
Lead researcher Krasi Vasilev from the University of South Australia's Future Industries Institute said current urinary diagnostic tests for urothelial cancer were expensive, with limited sensitivity and specificity.
"The thing with bladder cancer is the high probability of recurrence – about 75 per cent within five years, which is why they need constant surveillance," he said.
"Cystoscopy is an invasive procedure where you are at risk for a number of complications such as infections and with about 90 per cent of endoscopies being negative, patients can go away after having invasive procedures that cost a lot of money and are then told they are fine.
"This process will revolutionise how bladder cancer is tested and in the small tests we have done, it is better than other non-invasive techniques like urine cytology."
A research paper describing the technology was recently published online in Biosensors and Bioelectronics.
More information: Melanie Macgregor-Ramiasa et al. A platform for selective immuno-capture of cancer cells from urine, Biosensors and Bioelectronics (2017). DOI: 10.1016/j.bios.2017.02.011