A new approach to high insulin levels
Diabetes is characterised by a deficiency of insulin. Its opposite is a condition called congenital hyperinsulinism—patients produce the hormone too frequently and in excessive quantities, even if they haven't eaten any carbohydrates. Since the function of insulin is to metabolise sugars, excess production of insulin leads to chronic hypoglycaemia. The brain, which devours vast quantities of energy, is perpetually undernourished as a consequence.
The disorder can therefore lead to serious brain damage and even death in the worst cases. A team at the University of Geneva (UNIGE), Switzerland, supported by the Swiss National Science Foundation (SNSF) has succeeded in precisely describing the effects of a frequent genetic mutation in cases of congenital hyperinsulinism. This discovery, which has been published in Human Molecular Genetics, could pave the way for new therapies.
Congenital hyperinsulinism manifests symptoms from birth. Although it is considered to be a rare disease, affecting roughly one in every 50,000 newborn babies, it may be underdiagnosed. "Unless you are looking for it, hypoglycaemia can easily go unnoticed in an infant," explains Pierre Maechler, a researcher at the Faculty Diabetes Center at UNIGE and the lead author of the study. "Without intervention, it can rapidly take a dramatic course."
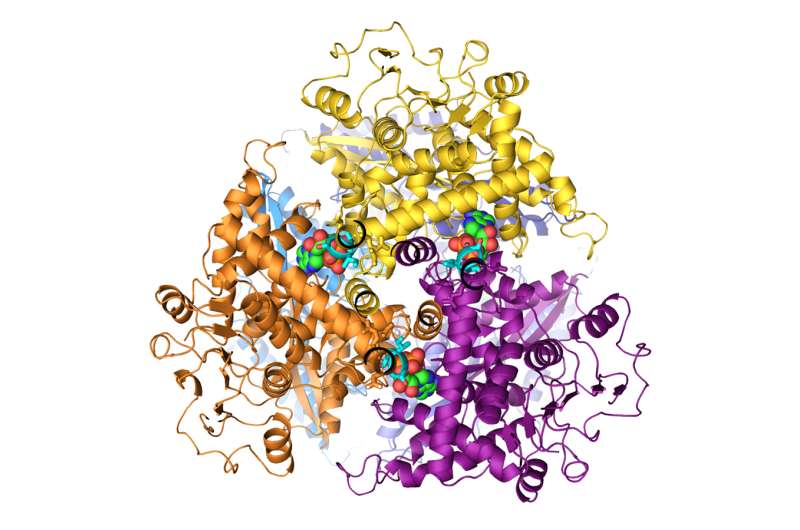
The researchers focused on a genetic mutation associated with hyperinsulinism. In healthy individuals, this gene produces a protein known as GDH, which opens up to receive a molecule known as an accelerator that binds to it. Then the protein moves into its active phase, sending a signal to the pancreas to produce more insulin until it reaches a certain threshold, after which GDH is no longer receptive to the accelerator molecule.
In congenital hyperinsulinism, the gene is mutated, changing the structure of the GDH protein. The protein remains permanently receptive to the accelerator molecule, whatever the level of glucose in the blood. As a result, it constantly sends signals to the pancreas to release insulin, which it produces in excess.
Insulin promotes the transfer of glucose to the muscles. If there is a constant surplus of insulin, it leads to undernourishment of the brain, which results in brain damage and intellectual retardation, and in extreme cases, coma and even death. Sugar is not the main culprit, though. "In these patients, even a meal consisting solely of protein will trigger the production of insulin," Pierre Maechler explains.
People with this mutation also develop a surplus of ammonia, a condition known as hyperammonaemia, which can have equally serious repercussions on brain function. This work, which was carried out by Ph.D. student Mariagrazia Grimaldi, showed that the cause of this problem is exactly the same—the mutant version of the GDH protein, which is always receptive to its accelerator, also causes excess production of ammonia in the liver.
The treatments currently available for congenital hyperinsulinism are problematic. They range from almost total removal of the pancreas, which induces diabetes artificially, to the administration of drugs regulating the activity of the pancreatic cells more or less precisely, but with major side effects.
This new study could pave the way for new treatments. "We can imagine developing a drug that inhibits the GDH accelerator by occupying the same site, which would reduce the production of insulin," Pierre Maechler says. A drug of this type might also be used to treat obesity. If there is no insulin in the body, the person does not gain weight. The researcher says, "The protein GDH could enable the production of insulin to be regulated. This type of approach, while appearing to offer an extremely simple solution, would, of course, raise questions and ethical problems. But we know that in some cases, diets don't work, and gastric bypass surgery is by no means a harmless solution, either."
The team working with Pierre Maechler is also studying the role of fructose in the development of type 2 diabetes. In a forthcoming publication, it shows that this sugar leads to glucose hypersensitivity, which manifests as increased insulin production. This discovery could confirm the suspected links between the massive use of fructose by the food industry since the 1980s and the sharp increase in the number of people with type 2 diabetes a few years later.
More information: Mariagrazia Grimaldi et al, Identification of the molecular dysfunction caused by glutamate dehydrogenase S445L mutation responsible for hyperinsulinism/hyperammonemia, Human Molecular Genetics (2017). DOI: 10.1093/hmg/ddx213