Timing could matter to how responsive cancer cells are to treatment, study suggests
DNA damage occurs routinely within your cells due to sun exposure, smoking and sometimes during the normal process of making new DNA. Fortunately, there are "checkpoints" in-place within cells to stop them from making more DNA and dividing before the damaged DNA is repaired. But in the case of cancer, cells may ignore these checkpoints, and go on to divide with damaged DNA.
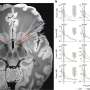
In a new study published in Cell Systems, University of North Carolina Lineberger Comprehensive Cancer Center researchers report that the timing of when DNA damage occurs within these different checkpoints matters to a cell's fate. They believe their findings could be helpful in predicting the outcome of cancer treatment with radiation and chemotherapy, which cause severe damage to cancer cells' DNA, triggering them to stop division, and die.
"It's very important to look at the timing of cell damage because depending on when it occurs within a certain cell cycle, there can be a totally different outcome," said Sherry Chao, the paper's first author and a graduate research assistant in the UNC School of Medicine Department of Genetics. "Some stages of the cell cycle are very vulnerable to DNA damage."
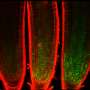
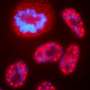
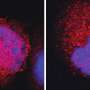
For the study, the researchers used time-lapse microscopy to study cells as they moved through three cell cycle phases, tracking the fate of individual cells after they received DNA damage. They found that the cells' response to DNA damage during certain cell cycle phases was strong and completely halted the cell cycle. In other phases, however, cells were relatively less sensitive or showed delayed but steady progression.
They also discovered that within cell cycle phases, there are "commitment points" past which cells will move forward into the next cycle, regardless of damage. "If the cell cycle has already passed through the commitment point, then the cell will continue with cell cycle progression," Chao said. "But if we damage the cells before they pass through the commitment point, the checkpoint is still able to stop the cell cycle."
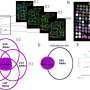
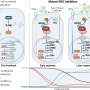
They used the data to build a mathematical model of how timing affects checkpoint behavior. The model suggests that the precise timing of DNA damage not only determines checkpoint behaviors, but also alters the cellular outcomes for damaged cells.
Researchers say the findings could inform new treatment strategies for administering common cancer therapeutics. Their findings suggest the possibility of synchronizing cancer cells in more susceptible cell cycle phases before treatment.
"Conceptually, it makes sense that if you could get all of the cells in the same cycle at the same time, you could damage them all with one shot," said UNC Lineberger's Jeremy Purvis, PhD, UNC Lineberger member and assistant professor in the UNC School of Medicine Department of Genetics. "The key will be finding ways to separate cancerous and healthy cells based on the timing and synchronization of their cell cycles."
Purvis added that their research adds the timing of treatment as an additional way of thinking about cancer research.
"If we can identify specific times when cancer cells will be more responsive to treatment, that's a fundamentally different approach than separating cells based on, say, a molecule you can detect on its surface."
More information: Hui Xiao Chao et al. Orchestration of DNA Damage Checkpoint Dynamics across the Human Cell Cycle, Cell Systems (2017). DOI: 10.1016/j.cels.2017.09.015