Study supports use of short-term HIV treatment interruption in clinical trials
A short-term pause in HIV treatment during a carefully monitored clinical trial does not lead to lasting expansion of the HIV reservoir nor cause irreversible damage to the immune system, new findings suggest.
Antiretroviral therapy (ART) benefits the health of people living with HIV, prolongs their lives and prevents transmission of the virus to others. If taken daily as directed, ART can reduce viral load—the amount of HIV in the blood—to levels that are undetectable with standard tests. However, the virus remains dormant in a small number of immune cells, and people living with HIV must take ART daily to keep the virus suppressed. If a person with ART-suppressed HIV stops taking medication, viral load will almost invariably rebound to high levels.
Researchers are working to develop therapeutic strategies to induce sustained ART-free remission—the absence of viral rebound following discontinuation of ART. Clinical trials to assess the efficacy of such experimental therapies may require participants to temporarily stop taking ART, an approach known as analytical treatment interruption, or ATI.
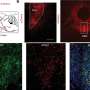
In the current study, researchers at the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, sought to better understand the immunologic and virologic effects of ATI. They analyzed blood samples from 10 volunteers who had participated in a clinical trial evaluating whether infusions of a broadly neutralizing antibody could control HIV in the absence of ART. During the trial, participants temporarily stopped taking ART. The 10 participants evaluated in the current study subsequently experienced viral rebound and resumed ART 22 to 115 days after stopping. While treatment was paused, the participants' HIV reservoirs expanded along with increases in viral load, and researchers observed abnormalities in the participants' immune cells. However, six to 12 months after the participants resumed ART, the size of the HIV reservoirs and the immune parameters returned to levels observed prior to ATI.
The findings support the use of ATI in clinical trials to evaluate the efficacy of therapeutic strategies aimed at achieving sustained ART-free remission, the authors conclude. However, larger studies that do not involve any interventional drugs are needed to confirm and expand on these results. The NIAID investigators currently are conducting a clinical trial to monitor the impacts of short-term ATI on a variety of immunologic and virologic parameters in people living with HIV.
More information: KE Clarridge, J Blazkova et al. Effect of analytical treatment interruption and reinitiation of antiretroviral therapy on HIV reservoirs and immunologic parameters in infected individuals. PLOS Pathogens DOI: 10.1371/journal.ppat.1006792 (2018).