Iron triggers dangerous infection in lung transplant patients, study finds
Researchers at the Stanford University School of Medicine have identified elevated tissue iron as a risk factor for life-threatening fungal infections in lung transplant recipients.
The study, to be reported Feb. 21 in Science Translational Medicine, investigated why lung transplant patients are more vulnerable to this fungus, Aspergillus fumigatus. "People rarely think about how a change in the patient's body tissues might make it better ground for invasion," said Mark Nicolls, MD, professor of pulmonary and critical care medicine and senior author of the study. Informed by this research, Nicolls has proposed a potential new approach for curbing these infections.
Aspergillus fumigatus is an extremely common mold, prevalent in even the most pristine hospital settings. "It's everywhere—we inhale thousands of these spores per day," said Joe Hsu, MD, assistant professor of medicine and lead author of the study. One-third of lung transplant recipients develop Aspergillus-related diseases, including severe asthma and often-fatal lower respiratory infections. The leading cause of death among these patients is transplant rejection, which the mold accelerates dramatically.
There are many risk factors for Aspergillus infection that are difficult to address in lung transplant recipients. Transplant patients are given medications that leave them less able to ward off infections, but without these drugs, their immune systems would attack their new lungs. During recovery, patients have no ability to cough up invading pathogens, making them especially vulnerable to the omnipresent spores. This is because the lung transplant procedure disrupts signals from the vagus nerve, which controls the cough reflex. Patients are usually put on antifungal medications in an attempt to prevent infection, but these pathogens are becoming more drug-resistant over time.
'Like fertilizer for Aspergillus'
All lung transplants carry these known risks, but not all lung transplants result in Aspergillus infections. Something causes the organism to behave differently in cases of infection, Hsu said. The study identified iron as a critical factor. "Iron is like fertilizer for theAspergillus," Nicolls said.
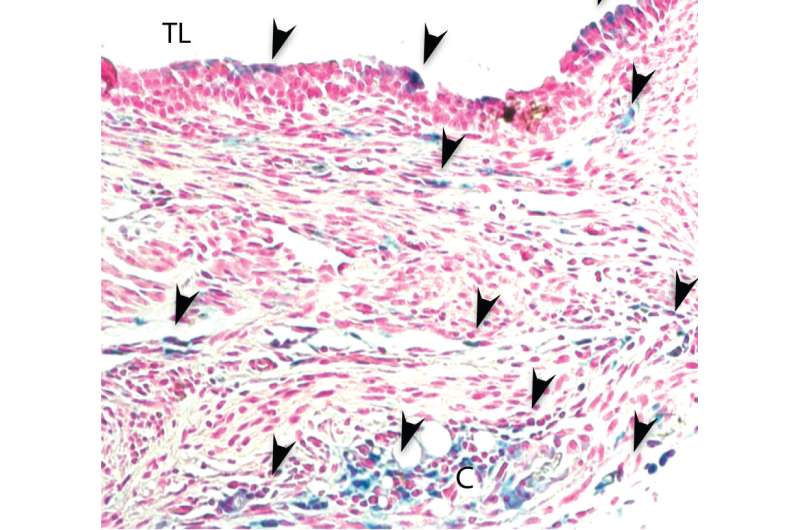
Hsu was interested in finding the Aspergillus trigger switch—the factor that prompts it to invade tissue. He studied the pathogen in mouse models by transplanting windpipes from one mouse to another. Observing the rejection process, he found that the transplanted tissue bled and accrued high levels of iron. Hsu biopsied human transplant patients and found the same distribution of iron, with higher levels in the transplanted tissue than the host. Thinking he may have found the trigger, Hsu introduced Aspergillus into the mice, comparing how it acted with and without access to iron.
He found that elevated iron prompted Aspergillus to invade. In experimental conditions that provided the pathogen more iron, it invaded the transplant. The more iron, the deeper the invasion progressed into the tissue. The results indicate increased iron is a major determinant of Aspergillus invasion. Differences in iron levels between transplant patients seem to explain why some become infected while others don't. "You could have lots of Aspergillus in the airway, which is fine because its everywhere, but it wouldn't penetrate the tissue unless there was iron beneath it," Nicolls said.
A potential treatment
The study suggests infection can be prevented by starving the Aspergillus organism of iron. Without iron to fuel it, the mold doesn't invade. The depth of invasion decreased in mice injected with an iron-reducing chemical. The result suggests a novel treatment route. Rather than targeting Aspergillus itself, doctors could modify tissue iron levels to change the organism's behavior, Hsu said. His next step is to study this treatment approach in humans, he said. High iron is characteristic of other pulmonary diseases, and Hsu predicts this methodology may be applicable across the field of pulmonary care.
Nicolls is an inventor on a new patent describing how lung transplant blood vessels may benefit from a low-iron environment, a property that could also include preventing mold invasion. He developed the technology with a number of collaborators, including Geoffrey Gurtner, MD, professor of surgery at Stanford, and Jayakumar Rajadas, PhD, director of Stanford's Biomaterials and Advanced Drug Delivery Lab, who is also a co-author of the study. The patent is a chemical solution that captures excess iron so the pathogen cannot use it. Nanoparticles act as a vehicle to transport the chemicals into the body.
"The capacity to deliver these compounds directly to the lungs is novel," Hsu said. "For the first time, we have a new way to treat these infections aside from antibiotics that try to wipe out the organism itself." The solution can be applied like a paint during surgery, inhaled into the lungs or injected intravenously.
The team's work is an example of Stanford Medicine's focus on precision health, the goal of which is to anticipate and prevent disease in the healthy and precisely diagnose and treat disease in the ill.
More information: J.L. Hsu el al., "Microhemorrhage-associated tissue iron enhances the risk for Aspergillus fumigatus invasion in a mouse model of airway transplantation," Science Translational Medicine (2018). stm.sciencemag.org/lookup/doi/ … scitranslmed.aag2616