Study: Absence of key protein, TTP, rapidly turns young bones old
The absence of a protein critical to the control of inflammation may lead to rapid and severe bone loss, according to a new University at Buffalo study.
The study found that when the gene needed to produce the protein tristetraprolin (TTP) is removed from healthy mice, the animals developed the bones of much older rodents.
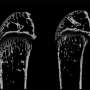
Within nine months, mice without the gene experienced a nearly 20 percent loss in oral bone. The results also revealed that overexpressing TTP in the animals led to a 13 percent reduction in bone turnover compared to unaffected mice.
Published on March 7 in the Journal of Dental Research, the study is the first to test TTP's influence on bone loss in an animal model.
Inflammation is a necessary reaction by the immune system to protect the body from injury or infection, but if not controlled, it can lead to the destruction of bone and the prevention of bone formation.
While TTP is known to play a major role in the regulation of inflammation, its production slows with age. The research results could have a profound impact on the management of bone health in the elderly, a population at higher risk of osteoporosis and periodontitis.
"TTP is the brake on the system. Without it, inflammation and bone loss would go unchecked," says Keith Kirkwood, DDS, PhD, lead author and professor in the Department of Oral Biology in UB's School of Dental Medicine.
"We don't know all of the reasons why TTP expression decreases with age. So, understanding the factors behind its expression and relationship with bone loss is the first step toward designing therapeutic approaches."
The researchers aim to advance their investigation toward similar studies in humans, particularly among the aging.
Osteoporosis, a condition in which bones become weak and brittle, and low bone mass affect nearly 55 percent of people age 50 and older, and it is estimated that by 2020, more than 61 million people will have either condition, the National Osteoporosis Foundation says.
The statistics surrounding periodontitis are equally grim. The infection - which damages the gums, destroys jaw bone and can lead to tooth loss - occurs in 70 percent of adults age 65 and older, according to the Centers for Disease Control and Prevention.
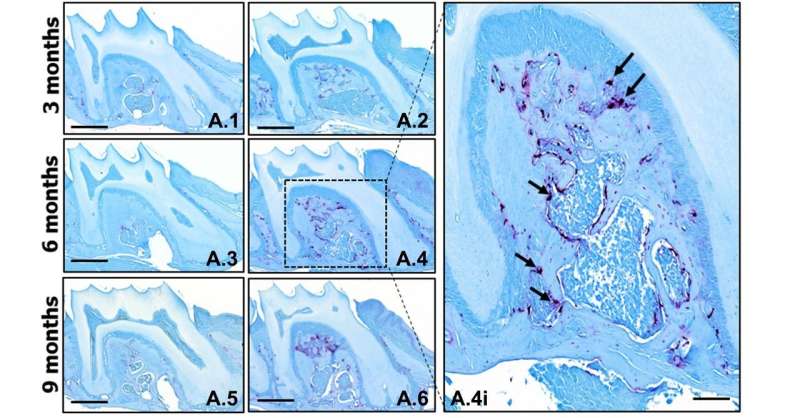
To better understand TTP's role in periodontitis, an inflammatory disease, the researchers studied three groups of healthy mice: a knockout group without the gene to express TTP, a knock-in group whose genes overexpressed TTP, and a control group of unaffected mice.
The rodents were tested for inflammatory conditions, oral bone levels and the presence of osteoclasts - cells that specialize in breaking down bone - in oral tissue at three-, six- and nine-month periods.
The researchers found that bone in the knockout mice aged more rapidly than in the control group. At three months old, the mice had lost 14 percent of their oral bone. By nine months - still a young age for a mouse - bone loss had increased to 19 percent.
In addition to periodontitis, the knockout mice developed arthritis, eczema and other inflammatory conditions. Osteoclast levels were also higher in the knockout group.
Investigators were surprised to find that the absence of TTP vastly altered the oral microbiome, despite all the rodents being housed in the same space. The finding suggests that systematic inflammation can affect the bacteria in the mouth. Further study is needed to determine whether the new bacteria are pathogenic or play a role in bone loss, says Kirkwood.
Overexpression of TTP in the knock-in mice increased protection against inflammation, lowering bone turnover by 13 percent. The increase in the protein had no effect on the number of osteoclasts, however.
A future investigation will study the effect of TTP on bone health over a two-year period. Kirkwood will also partner with Bruce Troen, MD, professor, and Kenneth Seldeen, PhD, research assistant professor, both in the Jacobs School of Medicine and Biomedical Sciences, to examine the differences in the protein's influence on oral bone and overall bone health.
More information: H.M. Steinkamp et al. Tristetraprolin Is Required for Alveolar Bone Homeostasis, Journal of Dental Research (2018). DOI: 10.1177/0022034518756889