Faulty cellular membrane 'mix' linked to Parkinson's disease
Working with lab-grown human brain cells, Johns Hopkins researchers report they have uncovered a much sought-after connection between one of the most common genetic mutations in Parkinson's disease and the formation of fatty plaques in the brain thought to contribute to the destruction of motor neurons that characterize the disease.
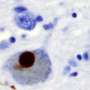
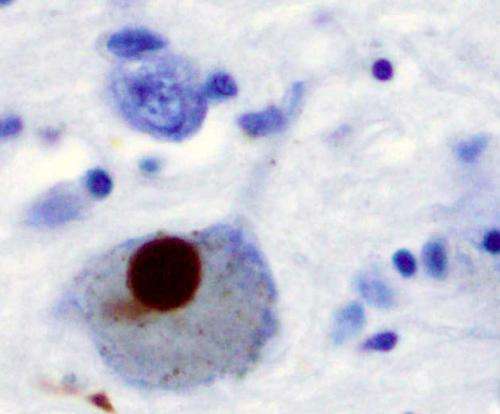
The mutation occurs in a gene that holds the code for GBA1, an enzyme that metabolizes fatty molecules in the cell, which make up most of brain cell membranes. The researchers believe that changes in the mixture of fatty molecules cause protein pieces to stick together in the brain, forming "dead zones" in the brain known as Lewy bodies, which can negatively affect movement, learning and behavior.
A summary of the work was published Jan. 8 in the Proceedings of the National Academy of Sciences.
"We believe this study gives us a better understanding of the effects of GBA1 mutation and its role in the development and progress of Parkinson's disease," says Han Seok Ko, Ph.D., associate professor of neurology at the Johns Hopkins University School of Medicine Institute for Cell Engineering.
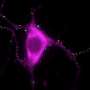
According to Ko, Lewy bodies are made of clumps of proteins known as α-synucleins. In healthy cells, single α-synuclein proteins tether together in groups of four, called tetramers, which are more resistant to aggregating in the brain. However, in Parkinson's disease, single α-synucleins stick together in the cell membranes, making it impossible for neurons to properly communicate with one another.
The cellular membrane is like a mosaic where fatty molecules act as the cement that holds proteins in an intricate design. In healthy cells, GBA1 ensures that the "cement" is mixed properly to hold the mosaic together. Ko says he and his team theorized that when GBA1 is mutated, this process goes awry and the cell membrane's composition is changed—determining whether the α-synuclein tetramers can stay in place.
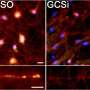
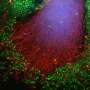
To test this theory, Ko and his team studied the effects of removing GBA1 in lab-grown human neuron cells using CRISPR-Cas9, a gene editing technology. They treated half of the "edited" cells with miglustat, a drug primarily used to block the production of fatty molecules, and observed the protein levels in the cell.
The researchers found that deleting GBA1 increased levels of a particular fatty molecule called glucosylceramide. When glucosylceramide levels rose, they reported, the number of stable α-synuclein tetramers fell. The levels returned to nearly normal with miglustat treatment.
Ko and his team believe that increased levels of glucosylceramide destabilized the cellular membrane mix and caused α-synuclein tetramers to fall out of the mosaic and break into single α-synucleins.
"This is interesting because past studies focused on how GBA1 mutations caused single α-synuclein aggregation, but not its effects on the stable tetramers," says Ko.
The researchers then tested their idea in human neurons collected from patients with GBA1-associated Parkinson's disease and found that—like the lab-grown cells—the human Parkinson's disease cells had about two times more glucosylceramide in their membranes than cells without the Parkinson's mutation. Similarly, the number of single α-synucleins also increased in these cells, and treatment with miglustat effectively restored α-synuclein tetramers to near-normal levels.
In a final test, the researchers wanted to investigate whether replacing GBA1 could restore the function of the membrane mix. After using a virus engineered to add a functional copy of the GBA1 gene into cells, Ko found that ?-synuclein tetramers returned to near-normal levels and the amount of α-synuclein aggregates was reduced.
In the future, the researchers intend to further investigate the impact the GBA1 protein has on the formation of α-synuclein tetramers and overall neuronal health.
First observed in 2004, GBA1-associated Parkinson's disease is the most common of the currently known Parkinson's disease mutations. According to the researchers, 5 to 10 percent of Parkinson's disease patients carry a GBA1 mutation.
More information: Sangjune Kim et al. GBA1 deficiency negatively affects physiological α-synuclein tetramers and related multimers, Proceedings of the National Academy of Sciences (2018). DOI: 10.1073/pnas.1700465115