Mitochondrial replacement moratorium should be reconsidered, researchers say
Mothers with mitochondrial DNA mutations often give birth to children who face incurable and fatal illnesses. But a much-studied form of mitochondrial replacement (MR) could prevent the transmission of such diseases from mothers to children, researchers say.
For that reason, two researchers argue that the U.S. moratorium that includes MR should be reconsidered through a process that engages the public, medical professionals, the U.S. Food and Drug Administration and Congress.
The authors—Eli Adashi, a professor of medical science at Brown University's Warren Alpert Medical School, and Harvard Law School professor I. Glenn Cohen—make their case in a March 2018 commentary in Obstetrics & Gynecology.
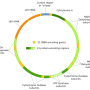
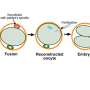
Such a process could clarify the benefits of the procedure—namely, the births of healthy children—and decouple it from misplaced concerns about genetic editing of embryos, the authors wrote. MR therapy simply replaces mutation-bearing mitochondria in oocytes (unfertilized, un-implanted eggs) with donated mutation-free mitochondria.
"A thousand children are born every year in the U.S. with serious, life-threatening issues that in a better world could be prevented by mitochondrial replacement," Adashi said. "While I have every respect for the sanctity of life, this issue is not about the sanctity of life. There is an inherent hypocrisy in holding this procedure hostage at the expense of 1,000 children each year who are doomed to die a painful death. There is nothing anti-life about the procedure, because no embryo is destroyed, and the life of baby is saved."
The moratorium
In 2016, legislation was passed that prohibits U.S.-based research in which a human embryo is intentionally created or modified, the study notes. While MR does not modify or "enhance" the nuclear genome, according to Adashi, replacing mutation-bearing mitochondria with donated mutation-free mitochondria falls under the general category of procedures prohibited by the moratorium.
In their commentary, Adashi and Cohen point out that the authors of the legislation are anonymous and that no congressional hearings, floor discussions or public engagement took place before its passage.
The authors surmise that the legislation may have been intended primarily to prevent embryo loss, a concern that does not apply to MR. A public process of reevaluating MR's inclusion in the moratorium could help to clarify that MR takes place before an embryo exists. The donated mitochondria are placed within unfertilized eggs, which can then be fertilized so that women can give birth to genetically related, disease-free children.
It is possible, Adashi said, that a misunderstanding of the sweeping nature of the legislation inadvertently bars the procedure.
"Mitochondrial replacement is best viewed as life-enhancing in its outlook by dint of its capacity to alleviate human suffering in a context where no other option exists," the authors wrote.
Impact of the moratorium
The moratorium deprives affected American families of the opportunity to prevent inherited, incurable and agonizing mitochondrial disease in their children, the authors contend.
Mitochondrial diseases include Leigh syndrome, a progressive and fatal disorder characterized by lesions on the brain that may lead to heart, kidney, vision and breathing complications, and Alpers Disease, a neurologic illness that causes seizures, dementia, spasticity, blindness, liver dysfunction and cerebral degeneration.
The moratorium may also induce American families to seek care outside of the country, according to Adashi and Cohen. They noted that a U.S.-led team in Mexico may have prevented Leigh syndrome in a child by replacing the mutation-bearing mitochondria of oocytes with donated mutation-free oocytes.
"This development calls into question the regulatory utility of a national moratorium in a globalized world wherein cross-border care is increasingly prevalent," Adashi and Cohen wrote in the study. It also creates risks, the authors assert, because there is no FDA oversight of these procedures that take place outside the U.S. border.
A path forward
Adashi and Cohen recommend that a coalition of patient and advocacy groups, medical professionals and legislators convene congressional hearings on the prevention of mitochondrial diseases. They also suggest convening a public meeting of the FDA's Cellular, Tissue and Gene Therapies Advisory Committee, charged with regulating reproductive technologies, to review the state-of-the-art procedure.
They also recommend stringent FDA oversight, the conditional approval of biologic licensing applications, clinic-specific licensing, possible sunset contingency provisions, and long-term intergenerational follow-up of the children of mothers who undergo mitochondrial replacement to determine the continuing safety and efficacy of the intervention.
In the U.K., a careful 15-year vetting process resulted in a vote in Parliament that approved MR under stringent regulatory oversight. In the U.S., on the other hand, "Congress legislated a statute that prohibits the FDA from adjudicating research into a range of procedures hereby treating the issue with a broad brush," Adashi said.
"They spent 15 years studying it—the science, the safety, the ethics—and they asked the British public what they thought," he added. "Now MR is legal but regulated by an agency that has been proceeding very cautiously, with just one clinic licensed to perform the procedure."
What this means, Adashi said, is that parents who are at risk for transmitting mitochondrial disease to their children may now undergo MR and have children who are not born with agonizing and untreatable diseases. American parents, Adashi and Cohen wrote in the commentary, deserve nothing less.
More information: Eli Y. Adashi et al, Preventing Mitochondrial Disease, Obstetrics & Gynecology (2018). DOI: 10.1097/AOG.0000000000002486