Dexcom wins FDA approval for next generation of glucose monitors for diabetes
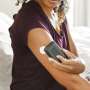
San Diego's Dexcom has won regulatory approval for its latest continuous glucose monitoring system that eliminates the need for finger pricks and is nearly one-third smaller than its current wearable sensor for diabetes patients.
The Food and Drug Administration this week gave the green light to Dexcom's G6 system for delivering real time blood sugar readings. Expected to hit the U.S. market in the second quarter, the G6 targets Type 1 and insulin-taking Type 2 diabetes patients.
"We think G6 carriers are a significant improvement in performance by building on the accuracy and reliability that patients have come to expect from Dexcom continuous glucose monitors," said Chief Executive Kevin Sayer in a conference call with analysts, "including what we believe will be a more consistent experience across all 10 days of use while eliminating the need for routine finger prick calibrations."
The approval was granted three months ahead of schedule, said Kyle Rose, an analyst with Canaccord Genuity. It also ushers in "meaningful product enhancements to help close any competitive gaps from new entrants Libre (from Abbott) and Guardian Connect (from Medtronic,)" said Rose.
Dexcom's shares gained 4.7 percent Wednesday to close at $73.28 on the Nasdaq exchange.
The G6 monitor is worn on the stomach. It contains a small sensor inserted just underneath the skin. The device wirelessly transmits glucose readings every five minutes to a smartphone, smartwatch or medical device app. It will trigger an alarm when a patient's blood sugar soars too high or drops too low.
Last fall, Abbott Labs received FDA approval for its Libre glucose monitory system for adults—challenging Dexcom's market leadership in the U.S. The Libre doesn't require finger pricks and sensors last for 10 days, compared with seven days for Dexcom's previous generation monitors, the G5, which needed finger stick calibration every day or two.
With FDA approval, Dexcom can now match Libre's features. In addition, Dexcom monitors continue to sound an alarm when patients experience blood sugar spikes or crashes—even while they're asleep.
The Libre does not automatically warn users when blood glucose reaches dangerous levels.
Dexcom declined to disclose the price for the G6 system. Insurance firms reimburse for continuous glucose monitoring for Type 1 diabetes, where high blood sugar levels can contribute to heart disease, stroke, blindness, kidney failure and nerve damage. Low blood sugar levels can also cause dizziness, unconsciousness and, in extreme cases, death. About 1.4 million Americans are living with Type 1 diabetes, based on research estimates.
Type 2 diabetes is less dangerous but more prevalent, afflicting as many as 27 million Americans. Insurance reimburses for glucose monitors for the most advanced Type 2 patients.
The FDA's approval for Dexcom's G6 monitor allows it to be used as a stand-alone sensor or in conjunction with insulin pumps and other diabetes management devices.
"The ability of this device to work with different types of compatible devices gives patients the flexibility to tailor their diabetes management tools to best meet personal preferences," said Donald St. Pierre, an acting director at the FDA's Center for Devices and Radiological Health.
Data from the G6 can be shared with up to five people, which helps parents of diabetic children keep track of blood sugar levels. And acetaminophen—the main ingredient in Tylenol—no longer interferes with the monitor's ability to deliver accurate readings.
"The G6 is a whole different platform, and we believe presents a major advancement for the continuous glucose monitoring category," said Sayer.
Baird Equity Research analyst Jeff Johnson said in a research note that less than 30 percent of Type 1 diabetes patients in the U.S. use continuous glucose monitors, where Dexcom is the clear technology leader in the market.
"Simply put, after focusing over the last year or two on concerns about Abbott and to a lesser extent Medtronic, we're starting to believe there are growing opportunities for Dexcom to widen its competitive advantages relative to these competitors," wrote Johnson.
©2018 The San Diego Union-Tribune
Distributed by Tribune Content Agency, LLC.