Following bats to predict Ebola
The 2014 Ebola outbreak in West Africa killed more than 11,000 people and was the deadliest outbreak since the discovery of the virus in 1976.
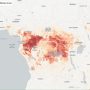
A New England Journal of Medicine study linked the start of the outbreak to a two-year-old in Meliandou, a remote village in Guinea, a country that had never before seen a case of Ebola. The type of virus was identified as the deadliest strain, found in Zaire—a country thousands of miles away.
How did it get there? The likeliest answer: bats.
A growing number of researchers are exploring the ecological dimensions that contribute to outbreaks of zoonotic diseases like Ebola that spill over from animal carriers to human populations. According to a report published by the Pulitzer Center, zoonotic diseases account for 60 percent of the roughly 400 emerging infectious diseases that have been identified since 1940. There is evidence that human activities such as deforestation are impacting animal migratory patterns as the search for resources is bringing animals into closer contact with humans.
Javier Buceta , associate professor of bioengineering and Paolo Bocchini, assistant professor of civil and environmental engineering have created a modeling framework that takes a zoonotic perspective on Ebola. The model considers the ecological dimensions that drive bat migration patterns and could help predict a bat-transmitted outbreak of Ebola among humans.
Now, the duo has been awarded a three-year grant from the National Institutes of Health (NIH) to further develop their modeling framework. The project, called "Risk Assessment of Ebola Outbreaks Through Probabilistic Modeling of Chiroptera Zoonotic Dynamics and Socioeconomic Factors, uses a novel sampling technique created by Bocchini that enables an analysis of spatially distributed random fluctuations of environmental parameters to understand how such factors impact bat migrations in a given region. The grant was awarded by the NIH's National Institute of General Medical Sciences.
The result of their work will be predictive tool to analyze the risk of Ebola outbreaks.
"Our framework shows that the appearance of Ebola outbreaks is tightly linked to fluctuations in environmental conditions which have an impact on both bat migration patterns and, therefore, infection rates," says Buceta.
This tool could be used to deliver guidance about the specific locations and periods of the year during which an outbreak is more likely to appear due to bats. It could also help reduce the risk of future spillovers from animals to humans.
"As a civil engineer," says Bocchini, "I look forward to closing the loop and studying how our society, our urban fabric and our infrastructure systems play a role in disease spreading in developing and developed countries."
Tracking the migratory patterns of bats
The team's approach works by tracking the migratory patterns of bats, which are believed to be a main carrier of the Ebola virus.
The model utilizes information on bat birth and death rates, the rate of infection of bats with the Ebola virus and recovery rates, bat mobility, seasonal changes and information about the availability of food and shelter to forecast bat infection peaks in a given region. That data is combined with a probabilistic modeling technique to predict the conditions linking bats' behavior with the outbreak of Ebola.
Establishing how to measure the key environmental factors driving resource-related bat migration has been key to developing the model.
Bocchini, a civil engineer, had been working with smart sampling techniques to resolve parameter fluctuations pertaining to his research on structural engineering and regional hazards. Through that work, he developed a highly efficient computational technique that addresses probabilistic big-data problems and enables researchers to analyze a small subset of truly representative cases.
With the support of NIH funding, the team will apply the "full force" of Bocchini's novel sampling technique, called Functional Quantization, and conduct extensive numerical simulations on a High Performance Computing platform. They will also incorporate socioeconomic, cultural and demographic information to understand how such factors impact Ebola transmission from bats in a given region.
The team's method demonstrates the importance of examining the big picture when trying to find answers to complex questions.
"It's clear that humans have our own role in the process," says Buceta. "Through our actions, we not only disrupt sensitive ecological systems, but also increase our own chance of becoming infected with serious—sometimes deadly—diseases."