Immunotherapy provides long-term survival benefit: Further evidence in lung cancer
Further evidence that immunotherapy provides long-term survival benefit for patients with lung cancer was presented today at ELCC 2018 (European Lung Cancer Congress) in Geneva, Switzerland.
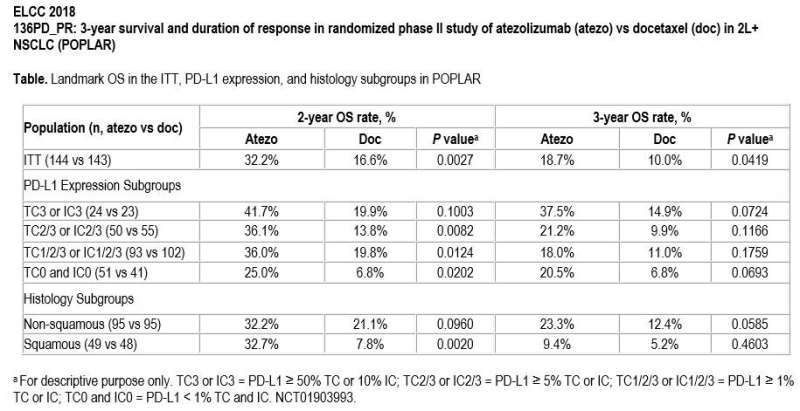
Researchers presented the three-year survival results of the randomised phase 2 POPLAR trial in second line, which is the longest follow-up reported to date with anti-programmed death ligand 1 (PD-L1) immunotherapy in patients with previously treated, advanced non-small-cell lung cancer (NSCLC). The trial randomised 287 patients from 61 sites across 13 countries with advanced NSCLC to the anti-PD-L1 antibody atezolizumab or docetaxel (chemotherapy).
Overall survival was significantly higher with atezolizumab at two and three years compared with docetaxel. Nearly one-third of patients (32.2%) in the atezolizumab treatment group were alive at two years compared with 16.6% in the docetaxel group. Additionally, at three years, almost twice as many patients (18.7%) were alive in the atezolizumab group compared to the docetaxel group (10.0%). The long-term overall survival benefit with atezolizumab over docetaxel was observed across histology (squamous and non-squamous) and regardless of PD-L1 expression. Even patients with PD-L1 expression in less than 1% of tumour cells and less than 1% of immune cells had a promising rate of long-term survival.
The median duration of response was three times longer with atezolizumab (22.3 months) compared to docetaxel (7.2 months). Atezolizumab led to fewer adverse events than docetaxel.
Lead author Dr Julien Mazières of Toulouse University Hospital, Toulouse, France, said: "Nearly one in five patients treated with atezolizumab was alive at three years. This places atezolizumab among the drugs with the highest landmark overall survival in previously treated lung cancer patients."
"The fact that all subgroups of patients benefitted to a similar degree is good in the sense that atezolizumab can be tried in all advanced NSCLC patients," he continued. "On the other hand, it means that we cannot predict which patients are most likely to live for three years. We need to find biomarkers to help us identify the long-term survivors with the drug."
Mazières said the drug was well tolerated which meant that patients can keep taking it for several years. He said: "Some of my patients who were in the atezolizumab treatment group are now long-term survivors with lung cancer. They are not cured, but they have survived, have a good quality of life, and have returned to work. With immunotherapy we now have a new type of patient: long-term survivors with lung cancer who can go back to a normal life."
Commenting on the study, Prof. Solange Peters, Head of Medical Oncology, Centre Hospitalier Universitaire Vaudois (CHUV), Lausanne, Switzerland, ESMO President-Elect said: "Before immunotherapy, the long-term survival of non-small-cell lung cancer patients was close to 0%. POPLAR supports the concept that long-term survival is possible with immunotherapy. The three-year survival results of POPLAR are consistent with the three- and five-year survival with the anti-PD-1 antibodies pembrolizumab and nivolumab, respectively, in phase 1 trials. These latest results are exciting because unlike the previous two trials, POPLAR was a large, randomised trial and provides convincing proof that long-term survival now exists in lung cancer."
Peters said there was now a strong argument that every patient with advanced NSCLC should receive immunotherapy. She said: "In the nivolumab phase 1 trial 15% of patients were alive at five years, which in cancer is usually considered being cured. We should offer all patients this one in six chance of five-year survival. However, this poses a financial challenge for healthcare systems."
To make this strategy sustainable, Peters said a method was needed to identify the patients who will not benefit from immunotherapy. She said: "That would enable us to treat only the patients with a high chance of long-term survival with immunotherapy. POPLAR shows that PD-L1 is not a useful biomarker to exclude patients from immunotherapy, since some patients with very low expression had an overall survival benefit. Rather than a single biomarker, I think it will be a signature of many biomarkers including tumour mutation burden that identifies the patients who should not be treated."
Peters said trials are needed to assess the ability of a combination of biomarkers to predict which patients with advanced NSCLC do, and do not, survive long-term with immunotherapy: "These trials should be conducted in patients with similar characteristics to the long-term survivors in the phase 1 and 2 trials with atezolizumab, pembrolizumab, and nivolumab. So the first step will be to describe these patients in terms of demographics, smoking history, tumour mutation burden, expression of immune genes, and PD-L1 expression. Focusing future studies on these patients will help us to discover a biomarker signature for use in clinical practice."
More information:
Abstract 136PD_PR "3-year survival and duration of response in randomized phase II study of atezolizumab (atezo) vs docetaxel (doc) in 2L+ NSCLC (POPLAR)": presented by Julien Mazières during the Poster Discussion session "Immunotherapy and next-generation TKIs: from second to frontline treatment" on Thursday, 12 April, 07:45 to 09:00 (CEST) in Room A.
Journal of Thoracic Oncology, Volume 13, Issue 4, Supplement, April 2018.