There's a better way to screen for cervical cancer
A new study in the Journal of the National Cancer Institute, published by Oxford University Press, indicates that high-quality cervical cancer screening can be done effectively using a completely automated approach. The researchers involved in the study indicate that automated technology could increase cervical screening coverage in underserved regions.
Cervical cancer is caused by persistent infection with carcinogenic human papillomaviruses (HPV). HPV vaccination to control transmission is the ultimate prevention strategy; however, vaccination remains a long-term solution due to still-limited coverage and the long latency between infection and cancer development
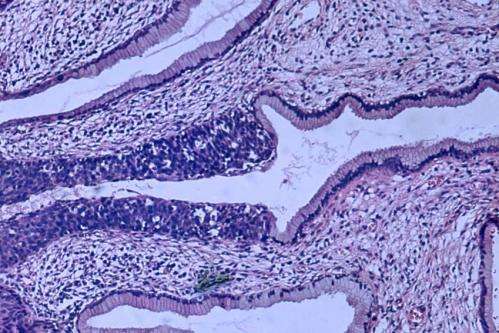
The main goal of cervical screening is to detect potentially premalignant conditions, which can be treated to prevent cervical cancer. Cervical screening programs include two procedures: screening of the general population and the separation of screen-positive women to focus treatment on potentially premalignant conditions. For the general screening phase, HPV testing for the carcinogenic types of HPV is gradually replacing cytology (Pap testing), because HPV testing is more sensitive for detection of precancer malignancies. It is also more reproducible and adaptable to increasingly HPV-vaccinated populations.
The leading treatment strategy in higher-resource regions combines partial HPV typing (to identify the highest-risk types) and Pap tests (used in this case as a second test among HPV-positive women). Conventional Pap tests, which are currently combined with HPV testing in most US screening programs to determine which women should receive treatment, is labor-intensive.
Use of computer-interpreted Pap tests for triage would permit the automation of the whole screening process. Here researchers report the design and evaluation of such a fully automatable cervical screening strategy to determine whether an automated algorithm could separate and prioritize HPV-positive women for treatment as accurately as conventionally interpreted Pap tests.
The researchers here developed a novel risk score algorithm based on computer-scanned liquid-based slide features to separate HPV-positive women to target potentially premalignant conditions using a slide scanner that performs a high-speed image capture to detect features of the Pap test slide such as the presence of different cell types, nuclear size, and nuclear contour. The severity rank is designed to identify the most innocuous slides in a batch, to reduce and/or guide treatment decisions.
Researchers compared it with abnormal Pap test results in predicting precancer among 1839 women testing HPV positive in 2010 at Kaiser Permanente Northern California. Precancer outcomes were ascertained by record linkage. As additional validation, they compared the algorithm with Pap test results among 243,807 women screened at Kaiser Permanente.
Among HPV-positive women, the algorithm matched the triage performance of abnormal Pap test results. Combined with HPV testing, the automated approach referred 91.7% of HPV-positive cases to immediate treatment while deferring 38.4% of HPV-positive women to one-year retesting (compared with 89.1% and 37.4%, respectively, for typing and Pap test triage). In the 2016-2017 validation, the predicted risk scores strongly correlated with Pap test results.
The results of this study showed that a computer algorithm matches or exceeds Pap test performance, suggesting that totally automated cervical screening without Pap tests is feasible and can improve cervical screening coverage in underserved regions.
More information: "Automated Cervical Screening and Triage, Based on HPV Testing and Computer-Interpreted Cytology," Journal Of The National Cancer Institute (2018). DOI: 10.1093/jnci/djy044