Taming an unruly target in diabetes
Focusing on a simple hormone in us all, a Yale researcher has found specific forms of it that poke toxic holes in cells—a discovery that he is leveraging into a treatment for patients with diabetes.
The research, published April 3 in Nature Communications, is also central to the recent awarding of two grants totaling $600,000 from the Connecticut Bioscience Innovation Fund and the Blavatnik Fund for Innovation at Yale.
Andrew Miranker, a professor of molecular biophysics and biochemistry and of chemical & environmental engineering, and his team will use these funds to translate the discoveries into novel therapies for type 2 diabetes. Part of this effort includes the formation of a new biotechnology company, ADM Therapeutics, based in Connecticut. Although the researchers are currently focusing on type 2 diabetes, the approaches they developed also apply to Alzheimer's and Parkinson's diseases.
Type 2 diabetes is a degenerative ailment that affects hundreds of millions of people worldwide. Its progression is tied directly to the health of insulin-producing cells in the islets—groups of cells in the pancreas. These cells carefully coordinate the release of insulin in response to changes in blood glucose. Failure of these cells plays a significant role in the cause of the disease as the body loses the ability to regulate blood glucose. Currently available drugs work by stimulating alternative ways for the body to use or eliminate glucose. There are no approved drugs available to address the causes of type 2 diabetes.
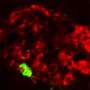
Miranker's lab is focused on a protein partner to insulin. The protein, known as islet amyloid polypeptide (IAPP), is also a hormone made by these same cells. The group has discovered that when IAPP adopts the wrong shape, it pokes holes in the membranes of islets large enough to kill the insulin-secreting cells.
"If we ameliorate these very large holes by designing a compound to target a particular IAPP structure, we can prevent toxicity," said Miranker.
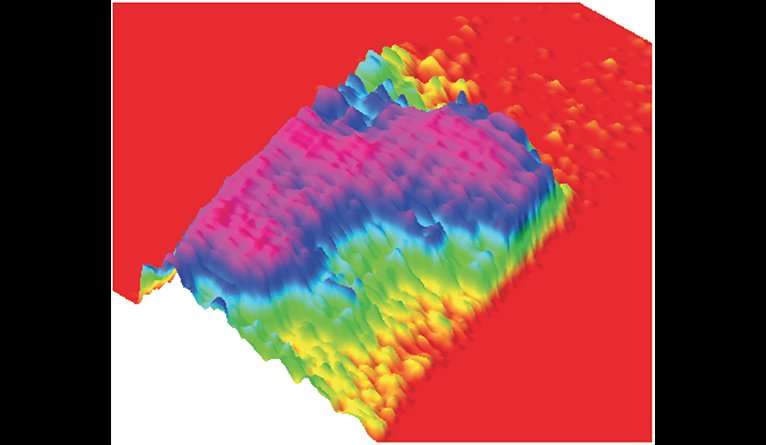
IAPP works alone in its healthy state, but the toxic version of IAPP is formed from tens to hundreds of copies of the protein. This sprawling structure poses a formidable challenge, note the researchers, and controlling it requires an approach very different from those of traditional drugs. Finding a drug to close a specific toxic hole should be a simple matter of finding the one square peg among round pegs, Miranker said, but mused, "What if your target is more like a porous pile of spaghetti than a hole?"
"Instead of thinking of a protein disease target as a rigid object with a well-defined pocket to aim at, you make the drug extremely well-defined and rigid, and you demand the protein adopt a structure to interact with it," Miranker said. This amounts to designing a drug that acts like a fork for the spaghetti to wrap around, he said, noting, "you can't eat spaghetti with a spoon."
To that end, the Miranker lab has developed a drug lead, ADM-116, that binds to IAPP and can rescue cells that make insulin. The water-soluble ADM-116 crosses the outer cell membrane, finds IAPP, and winds it up. By doing so, ADM-116 prevents IAPP from punching a hole in a sensitive internal cell membrane. Miranker and his team, and ultimately his Connecticut-based company, will translate these discoveries into drugs that improve the long-term health of these cells.
Miranker has been studying how changes in protein shape can result in toxicity for more than 20 years. Only now has this work reached a stage where his team can build off these fundamentals and apply it to human health. Miranker noted that the $500,000 grant from Connecticut Bioscience Innovation Fund, awarded in January, and last year's $100,000 grant from the Blavatnik Fund for Innovation at Yale were essential to making this leap.
Christopher Unsworth, associate director of business development for the Yale Office of Cooperative Research, said the promise of Miranker's research is that he takes such a different approach to the problem.
"His lab has developed a whole range of techniques to evaluate what is going on with these proteins and then designed a compound that could interfere with that process," he said. "A lot of times, we see basic research that identifies novel mechanisms that may be related to a disease, but understanding those mechanisms doesn't necessarily take you to a drug. Andrew's work, though, is directed pretty much toward chemical matter that could be a potential therapeutic."
More information: Conformational switching within dynamic oligomers underpins toxic gain-of-function by diabetes-associated amyloid. Nature Communications, DOI: 10.1038/s41467-018-03651-9