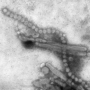
Early-stage respiratory syncytial virus (RSV) vaccine trial begins
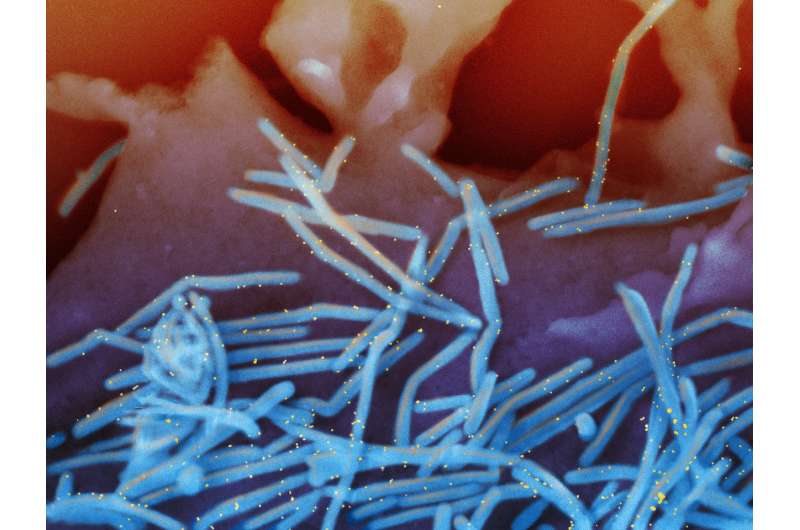
The National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health (NIH), has launched a clinical trial of an investigational vaccine designed to protect against respiratory syncytial virus (RSV). The Phase 1 study will enroll a small group of healthy adult volunteers to examine the safety of an experimental intranasal vaccine and its ability to induce an immune response. The study is being conducted at the Cincinnati Children's Hospital Medical Center, one of the NIAID-funded Vaccine and Treatment Evaluation Units (VTEUs).
RSV, a common virus, typically causes mild, cold-like symptoms that resolve within two weeks. However, the virus can cause severe symptoms, especially among infants and young children. Each year, an estimated 57,000 children under the age of five years are hospitalized in the United States due to RSV infection, according to the Centers for Disease Control and Prevention (CDC). Globally, RSV is estimated to cause up to 200,000 deaths annually, according to the World Health Organization. RSV is the most common cause of bronchiolitis (inflammation of the small airways in the lungs) and pneumonia among children under the age of 1 year. Almost all children in the United States are infected with RSV at least once by the age of 2, and most will experience repeated infections over their lifetimes.
Additionally, people 65 years or older, adults with chronic heart or lung disease, and individuals with weakened immune systems are at increased risk of severe RSV infection. Each year in the United States, RSV leads to an average of 14,000 deaths among adults older than 65 years, according to the CDC. Currently, no specific treatments or vaccines are available for RSV.
"RSV infection is a significant cause of illness and disease among the most vulnerable populations," said NIAID Director Anthony S. Fauci, M.D. "A vaccine to prevent disease from this pervasive and sometimes deadly virus is urgently needed."
Led by principal investigator David Bernstein, M.D., of the Cincinnati Children's Hospital Medical Center, the study is testing an experimental vaccine called SeVRSV. The vaccine candidate was developed by researchers at St. Jude Children's Research Hospital and manufactured by Children's GMP LLC, of St. Jude Children's Research Hospital in Memphis, Tennessee. SeVRSV contains a modified mouse virus (Sendai virus) designed to carry RSV genetic material that will express RSV fusion protein in the vaccine recipient to stimulate RSV-specific antibodies and T-cells. The Sendai virus vaccine platform has been well-tolerated to date in human clinical trials of vaccines for other infectious diseases, including HIV. SeVRSV performed well in previous preclinical and animal studies.
The study will enroll up to 25 healthy, non-pregnant volunteers between 18 and 45 years. After receiving an initial screening exam, at least 16 volunteers will be given a single intranasal dose of the experimental vaccine (0.22 milliliters in each nostril) and four or more will receive a placebo, in the form of saline nose drops. After vaccination, all volunteers will be observed for at least 30 minutes.
Afterwards, volunteers will report for five clinic visits over 29 days. During these visits, volunteers will be examined for any adverse reactions, and blood samples and nasal washes will be collected to check for RSV-specific antibodies. The volunteers will return at two and six months after vaccination.