Researchers find transport molecule has unexpected role

UT Southwestern researchers recently reported a basic science finding that might someday lead to better treatments for neurodegenerative diseases like a hereditary form of Lou Gehrig's disease.
In a study published in the journal Cell, Dr. Yuh Min Chook, Professor of Pharmacology and Biophysics, and Dr. Michael Rosen, Chair of Biophysics and an Investigator in the Howard Hughes Medical Institute (HHMI), are corresponding authors of the work in the emerging field of biological phase separation. This field encompasses aspects of the organization of the cell interior, including how proteins can phase separate like oil does from water. Dr. Chook, a Eugene McDermott Scholar in Medical Research, holds the Alfred and Mabel Gilman Chair in Molecular Pharmacology. Dr. Rosen, holds the Mar Nell and F. Andrew Bell Distinguished Chair in Biochemistry.
Their work identifies details in how molecules bind—or fail to bind—to each other for transport into the nucleus of nerve cells in the brain and spinal cord. Proper binding and transport appear essential for the protein named FUS (fused in sarcoma).
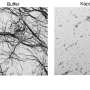
Normally, FUS binds to one of a class of nuclear transport receptors, in this case the importin molecule called karyopherin-β2 (Kapβ2), which transports proteins into the nucleus. Proper binding of FUS to Kapβ2 is necessary to take FUS into the nucleus, ensuring its correct cellular function. FUS that cannot be transported into the nucleus remains in the soupy cytoplasm of the cell and tends to self-associate (stick to itself) and form droplets of FUS suspended in cytoplasm that look like the droplets that form when a flask of oil and vinegar dressing is shaken.
Mutations in this transport system cause a genetic neurodegenerative disease called familial amyotrophic lateral sclerosis (fALS), also known as hereditary Lou Gehrig's disease.
The National Institutes of Health (NIH) describes ALS as a progressive disease that kills nerve cells controlling muscle movement. Death usually occurs within 10 years after symptom onset. An estimated 90 to 95 percent of ALS cases occur sporadically in people with no apparent family history. Several genes have been identified that, when deficient, underlie the familial form of the disease, which accounts for an estimated 5 to 10 percent of all ALS cases, according to the NIH.
In 2006, Dr. Chook identified a family of nuclear localization signals, dubbed the PY-NLS, used by the FUS protein. A PY-NLS sits at one end of the elongated FUS molecule and acts like a ZIP code for delivery of the FUS protein to the cell's nucleus. The NLS she identified became recognized as central to the development of fALS in which the FUS protein cargo cannot properly enter the nucleus and instead aggregates in the cell's cytoplasm surrounding the nucleus.
In 2012, Dr. Chook showed how the importin Kapβ2 recognizes the PY-NLS on the FUS protein and delivers it to the nucleus when the system is working properly. When the transport system fails, droplets of the FUS protein pile up in the cytoplasm, perhaps eventually becoming solid and toxic within the motor neurons of ALS patients.
Her discoveries allowed neuroscientists to figure out how FUS mutations lead to the aggregation of the FUS protein in the cytoplasm, but many questions remain unanswered. "The mutant FUS forms little liquid droplets within 10 minutes and these become less liquid and more solid over time, forming fibrils if the sample is left for 24 hours," Dr. Chook said.
"My laboratory has been working on phase separation, and Yuh Min's laboratory has done all this work on the FUS protein transport system. We wondered if the FUS droplets that we and others had observed would be affected by importin binding," Dr. Rosen said.
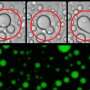
In studies of the purified FUS and Kapβ2 proteins, they found that the Kapβ2 blocks phase separation of FUS and that the importin's ability to block phase separation depends on its ability to read the ZIP code of the PY-NLS nuclear localization signal. When the ZIP code is unreadable, as in mutated FUS, phase separation into droplets continues unimpeded, the researchers said.
"If you add a small amount of Kapβ2 protein to FUS proteins that have formed into droplets, within five minutes the droplets disappear. So the importin Kapβ2 is playing two roles: It is the transporter but also protects the FUS protein against phase separation and aggregation," Dr. Chook said.
Using nuclear magnetic resonance (NMR) spectroscopy technology available in the Biophysics Department, the researchers next studied exactly how the Kapβ2 importin binds to the FUS protein. They found that under normal conditions, the Kapβ2 and FUS proteins bind very strongly to each other because of interactions at the nuclear localization signal—the ZIP code-like PY-NLS that sits at one end of the long FUS protein molecule. They also found that strong binding at the PY-NLS enables weaker and very brief interactions between other parts of the importin molecule and many other regions of the FUS protein.
"The NMR equipment is highly sensitive to time intervals, making it possible to 'see' the transient binding of the two molecules. Doing so would be impossible with a static system such as X-ray crystallography," Dr. Rosen explained.
"Binding at the right time, in the right place, in the right way is really important. All this transient binding appears to keep the FUS protein from interacting with itself and inhibits assembly into droplets," Dr. Chook said. "Those droplets are probably the initial stages of the aggregates that are a hallmark of familial ALS. If we could find a way to keep the droplets from forming, perhaps we could change the course of that neurodegenerative disease."
More information: Takuya Yoshizawa et al. Nuclear Import Receptor Inhibits Phase Separation of FUS through Binding to Multiple Sites, Cell (2018). DOI: 10.1016/j.cell.2018.03.003