AASM publishes clinical practice guideline on use of actigraphy for sleep disorders
Actigraphy can be a useful clinical tool for the evaluation of adult and pediatric patients with suspected sleep disorders, including circadian rhythm sleep-wake disorders, according to a clinical practice guideline from the American Academy of Sleep Medicine (AASM).
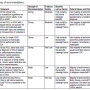
The guideline, which is published in the July 15 issue of the Journal of Clinical Sleep Medicine, provides recommendations for the use of actigraphy in adult and pediatric patients with suspected or diagnosed sleep disorders or circadian rhythm sleep-wake disorders. It includes seven conditional recommendations describing clinical scenarios in which clinicians can use actigraphy to help them understand a patient's sleep habits across multiple nights. Additionally, one strong recommendation indicates that clinicians should not use actigraphy in place of electromyography for the diagnosis of periodic limb movement disorder.
"Actigraphy is a clinical tool that has an important role in the assessment of certain sleep disorders, especially chronic insomnia and circadian-rhythm sleep-wake disorders," said lead author Michael T. Smith, MA, Ph.D., a professor of psychiatry and behavioral sciences at Johns Hopkins School of Medicine in Baltimore. "Actigraphic devices are easy for children and adults to use at home, allowing clinicians to gather objective data across multiple nights to gain a better understanding of a patient's typical sleep timing and duration."
Actigraphic devices typically are worn on the wrist or ankle for sleep assessment, and they use an accelerometer to record and integrate the occurrence and degree of limb movement activity over time. Mathematical algorithms are then applied to these data to estimate wakefulness and sleep. Potential benefits of actigraphy include its convenience, relatively low patient burden, longitudinal assessment capability, and relatively low cost.
Developed by an expert task force and approved by the AASM board of directors, the guideline updated previously published practice parameters and was based on a systematic literature review, meta-analyses, and assessment of the evidence using the GRADE methodology. A draft of the guideline was previously made available for public comment.
The systematic review focused exclusively on clinical grade devices approved by the U.S. Food and Drug Administration. It did not cover consumer wearable devices, mobile apps, or other non-prescription devices directly marketed to consumers, which were the subject of a recently published AASM position statement on consumer sleep technology.
"Actigraphy provides useful objective metrics across a variety of sleep-wake disorders in the setting of a comprehensive sleep evaluation at an accredited sleep center," said AASM President Dr. Douglas Kirsch. "However, it is important to recognize that actigraphy is not a substitute for polysomnography when clinical circumstances require a more detailed evaluation of sleep."
More information: Michael T. Smith et al, Use of Actigraphy for the Evaluation of Sleep Disorders and Circadian Rhythm Sleep-Wake Disorders: An American Academy of Sleep Medicine Clinical Practice Guideline, Journal of Clinical Sleep Medicine (2018). DOI: 10.5664/jcsm.7230