September 24, 2018 feature
Fusion hybrids: A newly discovered population of tumor cells
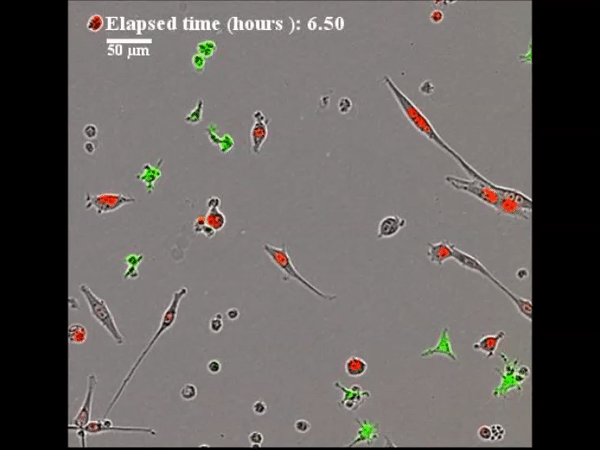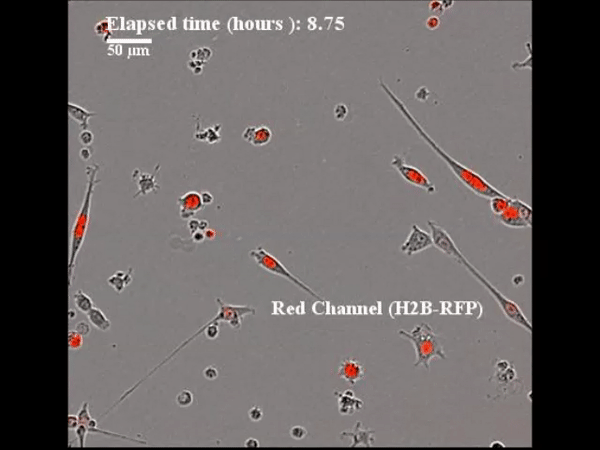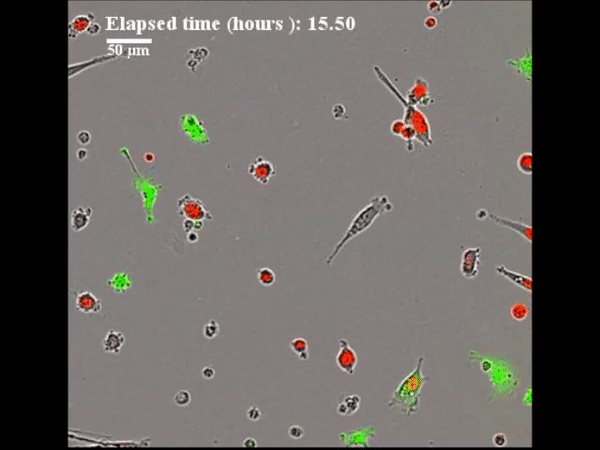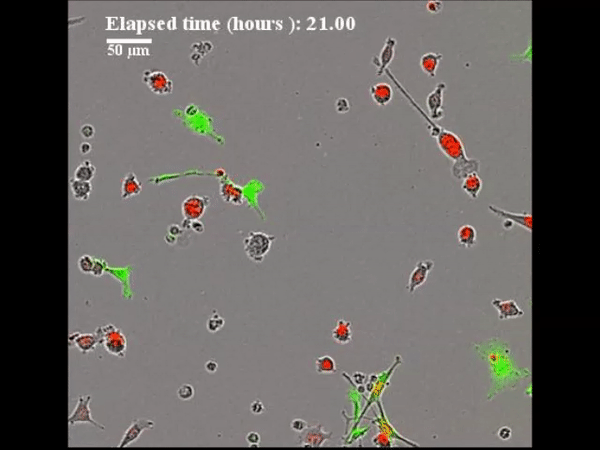
In a recent study published in Science Advances, Charles E. Gast and co-workers detail the spontaneous process of cancer cell fusion with white blood cells to produce heterogenous hybrid clones in multiple biological systems, including in mice and in humans. The authors identified a new population of tumor cells that may reveal insight into a potential therapeutic target for intervention in human cancer. Despite a century-old hypothesis that cell fusion contributes to tumor initiation and metastatic behaviour acquisition, only a few experimental studies have addressed the functional underpinnings of cell fusion in the etiology of malignant progression. Preceding biological studies have only circumstantially addressed the significance of hybrid tumor cells to support the mechanism. The underlying intracellular mechanisms cannot be easily identified or determined in human subjects, therefore, murine models and in vitro studies have provided an appropriate platform in the present study.
Previous work by the same research group reported in vivo fusion between the intestinal epithelial cells and macrophages (also referred to as MФs) in a cancer context. The results generated hybrid offspring that retained epithelial character as defined by their gene expression profile. As a follow-up, the authors presented a systematic analysis of MФ–neoplastic cell fusion (referred to as MФ–cancer cell fusion or fusion hybrid) with ex vivo and in vivo murine cancer models. The study provided evidence that hybrids acquired functional MФ-associated phenotypes to enhance tumor progression. Subsequent analysis of human tumor biopsies and peripheral blood revealed a novel circulating hybrid cell (CHC) population. These cells were defined to harbor hematopoietic and epithelial/tumor properties. The number of hybrid cells correlated with the disease stage and predicted the overall outcome, providing a biomarker for patient classification.
In the study, the authors generated in vitro-derived hybrids by engineering two mouse cancer cell lines: colon adenocarcinoma and melanoma (MC38 and B16F10). The results indicated that MФ-neoplastic cell fusion resulted in a variety of hybrid cells that shared features of both parental predecessors, while retaining their own characteristics.
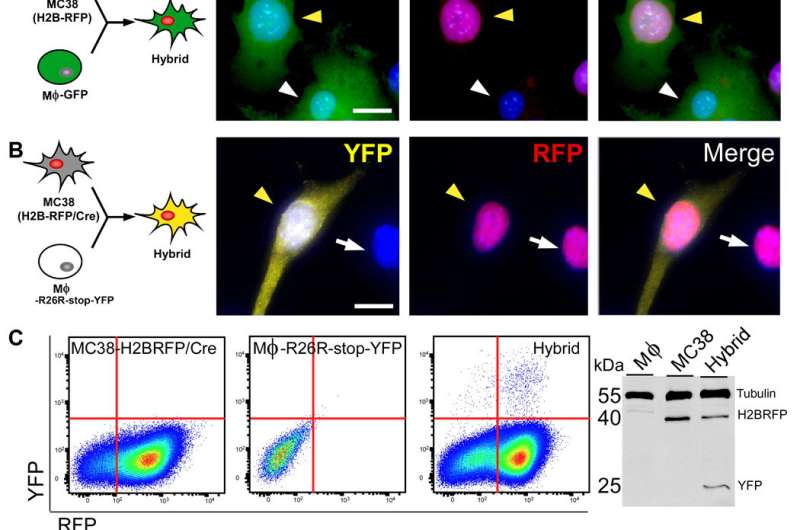
Next, the researchers studied cell fusion in mouse models of cancer after injecting 3000 in vivo-derived fusion hybrids into three mice. The results indicated that fusion hybrids showed different rates of tumor growth, suggesting the unique growth ability of hybrid cells and the ability to result in different rates of tumor growth.
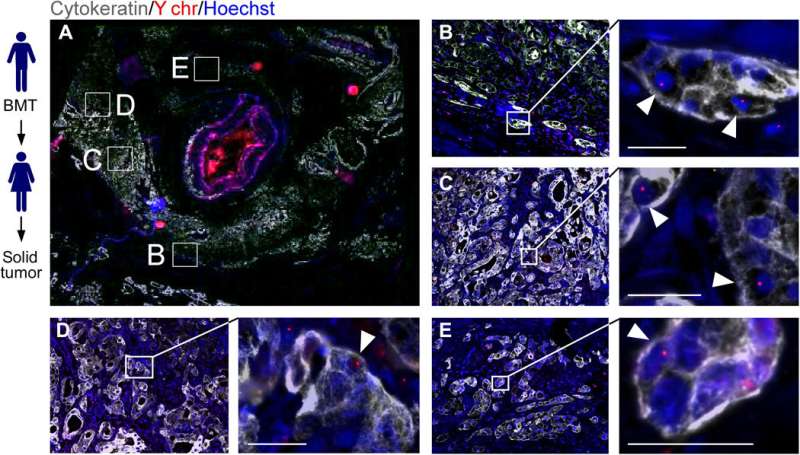
Gast and co-workers then analyzed tumor biopsies and peripheral blood from several female cancer patients to understand the biological significance of hybrids in humans. The researchers first determined if hybrids between blood cells and epithelial-derived cancer cells were detectable. The patients had previously received a sex-matched bone-marrow transplant that led to a second solid tumor, providing a disease scenario that supports the identification of hybrids harboring properties of blood cells and epithelial cells. The results indicated that all biopsies contained evidence of MФ-tumor cell hybrids that correlated with the disease stage and rate of patient survival.
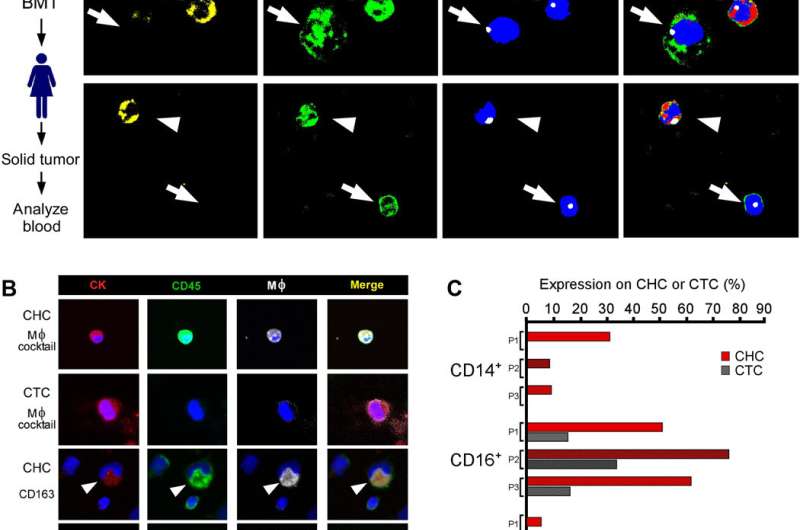
The findings identified a novel population of tumor cells in circulation (CHCs) that were previously overlooked and excluded from routine analysis. The findings introduce a functionally significant aspect of tumor progression and evolution to uncover a new area of tumor cell biology. As a chimera of MФs and neoplastic cells, the unique tumor population showed immune privilege in peripheral blood—a trait bestowed by their leukocyte identity. Understanding how such hybrids respond to immune therapies will offer an important area of investigation in the future. Together, the in vitro and in vivo data may reveal insight into diverse tumor cell pathophysiology underlying the treatment resistance, tumor progression and recurrence post-treatment in human cancer.
More information: Charles E. Gast et al. Cell fusion potentiates tumor heterogeneity and reveals circulating hybrid cells that correlate with stage and survival, Science Advances (2018). DOI: 10.1126/sciadv.aat7828
Anne E. Powell et al. Fusion between Intestinal Epithelial Cells and Macrophages in a Cancer Context Results in Nuclear Reprogramming, Cancer Research (2011). DOI: 10.1158/0008-5472.CAN-10-3223
Naohiro Terada et al. Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion, Nature (2002). DOI: 10.1038/nature730
© 2018 Medical Xpress


















