First-of-its-kind research models immune responses in cellular immunotherapies

In the Cellular Immunotherapy and Transplantation Program at Virginia Commonwealth University Massey Cancer Center, scientists are pursuing a cross-collaborative effort that could potentially change the way cellular immunotherapies such as stem cell transplantation and CAR T-cell therapies are performed. This grassroots research is funded primarily through VCU Massey pilot grants, and it is culminating in a first-of-its-kind body of work that provides a detailed quantitative view of how the immune system responds to cellular therapies.
"We have developed the mathematical basis of the immune responses observed in stem cell transplantation and other forms of cancer therapy reliant upon the immune system," says Massey hematologist-oncologist and lead investigator Amir Toor, M.D. "This has allowed us to understand the immune responses that occur following transplantation and cancer immunotherapy as a deterministic, rather than random process. In the future, we hope such models will be used to facilitate treatment decisions, which will improve the outcome of patients treated for cancers of the blood and lymphatic system."
Toor has been pursuing the idea of modeling immune responses since he discovered fractal patterns in the development of T-cells when he examined DNA sequencing data from stem cell transplant donors and recipients more than 6 years ago. He gave a TEDxRVA talk about their discoveries in 2013.
But in the past year, Toor and his team have published three additional studies that are shedding more light onto the seemingly random nature of immune responses and taking them further in their ability to personalize treatments in order to avoid potentially life-threatening complications such as graft-versus-host disease (GVHD) in stem cell transplant patients and cytokine release syndrome in CAR T-cell patients.
The first of the three studies, published in the journal PLoS ONE, analyzed DNA sequencing samples from 27 HLA-matched related and 50 unrelated stem cell donors and transplant recipients, and it found substantial differences in protein coding between the two.
The human leucocyte antigen (HLA) refers to the genes that encode for proteins on the surface of cells that are responsible for regulating the immune system. Doctors currently use HLA testing to match stem cell donors and recipients who have similar HLA makeup, and it identifies the major HLA genes a person has inherited and their corresponding antigens. The proteins produced by HLA genes help the body's immune system distinguish which cells are "self" and those that are foreign.
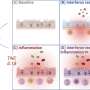
The researchers looked at variation in minor histocompatibility antigens (mHA) of stem cell transplant donor-recipient pairs. The mHA are the protein fragments presented on HLA molecules, which interact with receptors on immune system T-cells. Using advanced computer-based analysis, the researchers examined potential interactions between the mHAs and the HLA and discovered a high level of mHA variation in matched donor-related pairs. This variation could contribute to graft-versus-host disease (GVHD), a potentially life-threatening complication in which the donor's immune cells attack healthy tissue in the recipient following transplantation.
Using the computer models they developed based on mHA interactions and protein coding differences between the donors and recipients, the scientists confirmed that the magnitude of simulated T-cell response in their models correlated with cumulative GVHD incidence in the patients.
"We believe this type of modeling could be used to identify optimal donors for transplant recipients, reducing the risk of complications such as graft-versus-host disease. Alternatively, it may also inform immunosuppression dosing following transplantation," says Toor, who is also a member of the Developmental Therapeutics research program at Massey.
In the second study, recently published in the journal Bone Marrow Transplantation, Toor applied the model developed in the first study to chimeric antigen receptor (CAR) T-cell therapy, a new type of immunotherapy in which a patient's own T-cells are extracted and genetically engineered to attack a patient's specific type of cancer.
Currently, CAR T-cell therapy dosing is similar to donor lymphocyte infusion (DLI) dosing for stem cell transplantation. These models are inherently flawed because DLI dosing fails to take into account the unique action of CAR T-cells.
CAR T-cells are designed to respond to a specific antigen presented on the cancer cells and grow exponentially in reaction, reaching a peak population of T-cells before plateauing. The magnitude of this response is proportional to the level of antigen expression, which is directly related to the disease burden of the patients. Different binding affinities of the CAR construct may also influence the effectiveness and toxicity of the therapy.
In the study, researchers presented a mathematical formula that may be used to develop dosing parameters that account for these variabilities. Precisely calculating the CAR T-cell therapy dose may help limit the severity of cytokine release syndrome, a potentially fatal inflammatory immune response resulting from the interaction between the CAR T-cells and the cancer cells.
"We demonstrated that it is possible to mathematically derive a relationship between CAR T-cell expansion and the target leukemia cell growth," says Toor. "This will help doctors establish dosing parameters specific to a patient's tumor burden that limit toxicity."
The final study, recently published in the journal Frontiers in Immunology, takes the previous models even further. Toor and his team accounted for additional variables present in stem cell transplantation, which may help permit more accurate simulation of T-cell responses between donors and recipients.
In the study, whole exome sequencing was performed on previously cryopreserved DNA samples from 77 HLA-matched donor-recipient pairs. Using this data, the researchers identified a vast array of antigens that may be presented on different HLA molecules in each patient. It also highlighted additional factors that can potentially influence treatment outcomes such as the binding affinities for the antigens and their corresponding T-cell receptors.
"From this research, we have developed a comprehensive mathematical framework of immune response that shines a new light on various factors critical to T-cell immunology. These models firmly establish that immune responses are not random, but are amenable to computation and prediction," says Toor. "Our work represents a major conceptual breakthrough in applying precision medicine to stem cell transplantation and cancer immunotherapy."
Ultimately, Toor and his colleagues hope to use these models in combination with DNA sequencing to improve outcomes from cellular therapies by optimizing donor-recipient matching, CAR T-cell dosing and interventions for side effects such as graft-versus-host disease and cytokine release syndrome.
The researchers are continuing to refine their models while working to secure funding that will allow them to validate their findings by using whole exome sequencing to evaluate and treat patients undergoing cellular therapies at Massey Cancer Center. Their future work will study how these DNA sequence repertoire differences may change the donor immune cell profile after transplantation, molding it to the recipient's tissue antigen profile. This research finding will give physicians a real-time ability to measure immune responses and make appropriate adjustments in treatment.
More information: Ali Salman et al, Determining the Quantitative Principles of T Cell Response to Antigenic Disparity in Stem Cell Transplantation, Frontiers in Immunology (2018). DOI: 10.3389/fimmu.2018.02284