Inflammation-driven deterioration of structural proteins contributes to aging
Aging-related inflammation can drive the decline of a critical structural protein called lamin-B1, which contributes to diminished immune function in the thymus, according to research from Carnegie's Sibiao Yue, Xiaobin Zheng, and Yixian Zheng published in Aging Cell.
Each of our cells is undergirded by a protein-based cellular skeleton. And each of our tissues is likewise supported by a protein matrix holding the cells that comprise it together. These protein scaffolds or structures are necessary for organs and tissues to be constructed during development.
"Since organ building and maintenance require this protein-based structural support system, we thought it was natural to ask whether age-related deterioration of the architectural proteins would contribute to the degeneration of the organ as a whole," Zheng explained.
One of the major families of structural proteins are called lamins. They are highly evolutionarily conserved and found in an array of organs and tissues. These filamentous proteins are a major component of the meshwork that supports and organizes a cell's nucleus—where the genetic material is stored—and they influence gene expression during organ development and throughout life. Mutations in lamins are linked to developmental defects, premature aging, heart defects, and age-associated organ decay.
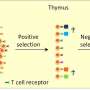
Yue, Zheng, and Zheng set out to understand the role that lamins play in aging tissue by studying special cells in the thymus that are essential for its development, organization, and function. As part of our body's immune system, the thymus makes T cells that function like soldiers to seek and destroy foreign invaders and fight diseases.
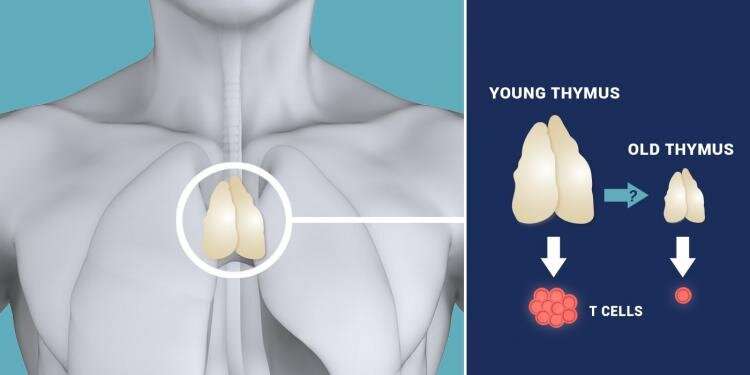
In almost all vertebrate species, the thymus is known to shrink and experience architectural changes with age, accompanied by diminished function.
"Since the thymic epithelial cells are essential for thymus development and function, they offer us a great model for studying how changes in lamins in these cells may contribute to the aging process," Zheng said.
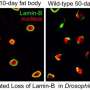
The researchers found that one member of the family, called lamin-B1, is required for thymic epithelial cells to develop and maintain normal function. What's more, they demonstrated that a reduction of lamin-B1 in the thymic epithelial cells is triggered by an age-associated increase in inflammation. This drives gradual deterioration of the thymus, eventually leading to organ dysfunction.
"We found a connection between a phenomenon that was already known—age-related inflammation in the thymus—and the deterioration of a key structural protein," Zheng said.
Looking forward, the team is interested in probing how this age-related decline in lamin-B1 impacts the crucial role that these architectural proteins play in maintaining gene expression.
More information: Sibiao Yue et al. Cell‐type‐specific role of lamin‐B1 in thymus development and its inflammation‐driven reduction in thymus aging, Aging Cell (2019). DOI: 10.1111/acel.12952