Study details N439K variant of SARS-CoV-2
An international team of researchers has characterized the effect and molecular mechanisms of an amino acid change in the SARS-CoV-2 Spike protein N439K. Viruses with this mutation are both common and rapidly spreading around the globe. The peer reviewed version of the study appears January 25 in the journal Cell.
Investigators found that viruses carrying this mutation are similar to the wild-type virus in their virulence and ability to spread but can bind to the human angiotensin converting enzyme 2 (ACE2) receptor more strongly. Importantly, researchers show that this mutation confers resistance to some individual's serum antibodies and against many neutralizing monoclonal antibodies, including one that is part of a treatment authorized for emergency use by the U.S. Food and Drug Administration.
"This means that the virus has many ways to alter the immunodominant domain to evade immunity while retaining the ability to infect and cause disease," says senior author Gyorgy Snell, Senior Director of Structural Biology at Vir Biotechnology. "A significant finding from this paper is the extent of variability found in the immunodominant receptor binding motif (RBM) on the spike protein."
Although the recently emerged UK variant, B.1.1.7, and the South African variant, B.1.351, have garnered more attention to date, the N439K mutation is the second most common in the receptor binding domain (RBD). The N439K mutation was first detected in Scotland in March 2020 and since then, a second lineage (B.1.258) has independently emerged in other European countries, which, by January 2021, was detected in more than 30 countries across the globe.
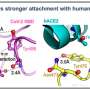
The Cell study also reports the X-ray crystal structure of the N439K RBD. "Our structural analysis demonstrates that this new mutation introduces an additional interaction between the virus and the ACE2 receptor," Snell says. "A single amino acid change (asparagine to lysine) enables the formation of a new point of contact with the ACE2 receptor, in line with the measured two-fold increase in binding affinity. Therefore, the mutation both improves interaction with the viral receptor ACE2 and evades antibody-mediated immunity."
Once researchers determined that the N439K mutation did not change virus replication, they studied whether it allowed evasion of antibody-mediated immunity by analyzing the binding of more than 440 polyclonal sera samples and more than 140 monoclonal antibodies from recovered patients. They found that binding of a proportion of both monoclonal antibodies and serum samples were significantly diminished by N439K. Importantly, the N439K mutation allowed pseudoviruses to resist neutralization by a monoclonal antibody that has been approved by the FDA for emergency use as part of a two-antibody cocktail. One way around this problem, researchers say, could be the use of antibodies that target highly conserved sites on the RBD. "The virus is evolving on multiple fronts to try to evade the antibody response," Snell says.
He notes that one of the challenges in studying SARS-CoV-2 variants is the limited amount of sequencing that's currently being done overall: more than 90 million cases of COVID-19 have been recorded and only about 350,000 virus variants have been sequenced. "That's only 0.4%—just the tip of the iceberg," he says. "This underscores the need for broad surveillance, a detailed understanding of the molecular mechanisms of the mutations, and for the development of therapies with a high barrier to resistance against variants circulating today and those that will emerge in the future."
More information: Cell, Thomson et al.: "Circulating SARS-CoV-2 spike N439K variants maintain fitness while evading antibody-mediated immunity" www.cell.com/cell/fulltext/S0092-8674(21)00080-5 , DOI: 10.1016/j.cell.2021.01.037