Neuromuscular disease registry helps patients access research, clinical trials, new genetic tests, and therapies

The Canadian Neuromuscular Disease Registry (CNDR) was launched in 2010 to increase efficient patient access to cutting-edge research and clinical trials, to increase understanding of the natural history and epidemiology of neuromuscular disease across Canada, and to facilitate research collaboration. An assessment of CNDR's accomplishments, published in the Journal of Neuromuscular Diseases, found that it has been successful in securing funding and engaging the community over the past 10 years. With more than 4,000 enrolled patients, data from the registry have been used in over 125 research projects as of 2019, including clinical trial and research notifications, patient questionnaires, and data analyses around patient outcomes and care.
"I am very proud of the work that has been done by the registry team and all of our collaborators across Canada," says lead author Lawrence Korngut, MD, MSc, Department of Clinical Neurosciences, and Hotchkiss Brain Institute, University of Calgary, Calgary, AB, Canada. "It takes a great deal of effort to enter high-quality data and it has been very fulfilling to provide use of the data currently more than 150 times in projects for researchers and organizations to benefit the neuromuscular community through positive impact on outcomes and advocacy."
Neuromuscular disease is a broad classification that includes numerous conditions that are individually rare, but collectively common, with a prevalence of 160 patients per 100,000 people. Canada is a very diverse country with a broad geographic spread and two national languages. With limited patient numbers in any individual clinic, the multi-disease methodology employed by the CNDR is an efficient and comprehensive way to facilitate collaboration across the country.
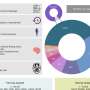
Diagnosis and contact information are collected from patients for all diseases. More detailed information, including a full disease-specific clinical dataset and patient demographics, is collected from individuals with one of five specific diseases: Amyotrophic Lateral Sclerosis (ALS); Duchenne Muscular Dystrophy (DMD); Myotonic Dystrophy (DM); Limb Girdle Muscular Dystrophy (LGMD); and Spinal Muscular Atrophy (SMA). For these indexed diseases, CNDR estimates that the registry has thus far ascertained variable percentages of the estimated total patient population in Canada, ranging from 9.0% for DM patients to 83.5% of DMD patients.
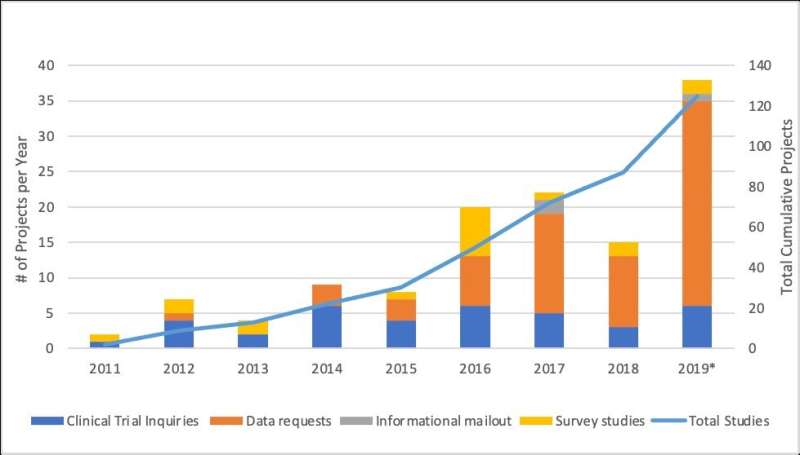
Among the projects facilitated by CNDR are more than 18 questionnaire studies, including quality of life studies for DMD and SMA patients, access to computer technology for DM patients, and a global study of risk factors for ALS. Organized to encourage companies to choose Canada for clinical trials and help recruit patients for clinical trials, it has also facilitated 37 clinical trial inquiries. Data have also been used to evaluate patients' access to care in different regions of the country and has helped the network of investigators advocate for their patients in access to therapy and care and support services.
As the current therapeutic landscape for neuromuscular disease is evolving, so too are the aims and scope of the CNDR. The registry has collected genetic test results ranging from 16.9% (of registered sporadic ALS patients) to 86.4% (of registered DM patients). Between 8.3% (LGMD) and 28.4% (DMD) of registrants have participated in clinical trials. The registry is now also being used to understand safety and effectiveness of new therapies.
"CNDR has been instrumental in encouraging clinics to assist registered patients in obtaining proper genetic testing," notes Dr. Korngut. "As there has been an increase in the number of disorders with available genetic testing and genetic-based therapies, awareness of and access to formal genetic counseling are essential components of clinician-patient discussions."
Dr. Korngut observes that in this new era of therapeutic discovery for rare neuromuscular diseases, national disease registries and their associated networks of affiliated clinics are essential. "Ultimately, the availability of comprehensive real-world data in Canada has been an asset to all stakeholders in the neuromuscular disease community and will likely become more so."
More information: V. Hodgkinson et al, The Canadian Neuromuscular Disease Registry 2010–2019: A Decade of Facilitating Clinical Research Through a Nationwide, Pan-Neuromuscular Disease Registry, Journal of Neuromuscular Diseases (2020). DOI: 10.3233/JND-200538