HIV vaccine candidate's mysteries unlocked 20 years later
About two decades after first devising a new kind of vaccine, Oregon Health & Science University researchers are unlocking why it stops and ultimately clears the monkey form of HIV, called SIV, in about half of nonhuman primates—and why it's a promising candidate to stop HIV in people.
In scientific papers that were simultaneously published today in the journals Science and Science Immunology, creators of the cytomegalovirus, or CMV, vaccine platform describe the unusual biological mechanisms through which it works.
The findings also helped fine-tune VIR-1111, the CMV-based experimental vaccine against HIV that was developed at OHSU and is now being evaluated in a Phase 1 clinical trial. The trial is being conducted by Vir Biotechnology, which licensed the CMV vaccine platform technology from OHSU.
"Knowing the mechanism that the CMV-based SIV vaccine uses to work in rhesus macaques gives us a way to judge the potential of a human vaccine very quickly," said Louis Picker, M.D., the associate director of the OHSU Vaccine and Gene Therapy Institute and a professor of pathology/molecular microbiology and immunology in the OHSU School of Medicine. "If you have the wrong genes in the CMV vaccine, the critical immune response needed for efficacy won't develop. You have to thread the CMV vaccine's needle exactly if you want a high degree of protection, and you have to know what you're looking for."
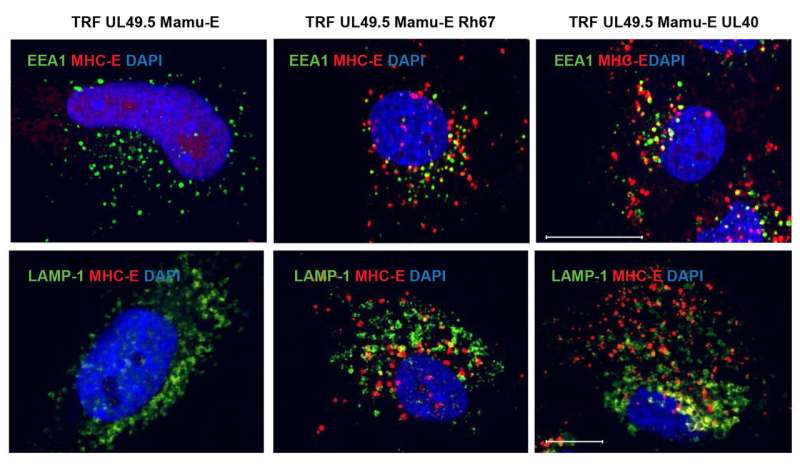
Two of the papers describe that the cytomegalovirus vaccine needs to generate an unusual type of CD8-positive T cell response called MHC-E-restricted T cells to effectively fight off SIV in monkeys.
"We knew for a while that we have unusual T cell responses in monkeys that receive our CMV vaccine against SIV," said Klaus Frueh, Ph.D., a professor of molecular microbiology and immunology in the OHSU School of Medicine and OHSU Vaccine and Gene Therapy Institute. "But we didn't know if they were important for protection against SIV. This research shows clearly that, without this special MHC-E-restricted T cell response, we don't have protection."
The study published in Science Immunology shows that the vaccine was only able to generate these special T cells to fight off SIV if eight specific genes were missing or inactivated from the natural form of monkey CMV. And a corresponding paper published via Science's First Release describes how a specific cytomegalovirus protein known as Rh67 is required to generate MHC-E-restricted T cells to protect against SIV. Together, these papers suggest how a CMV-based vaccine needs to be designed to create these unconventional T cell responses.
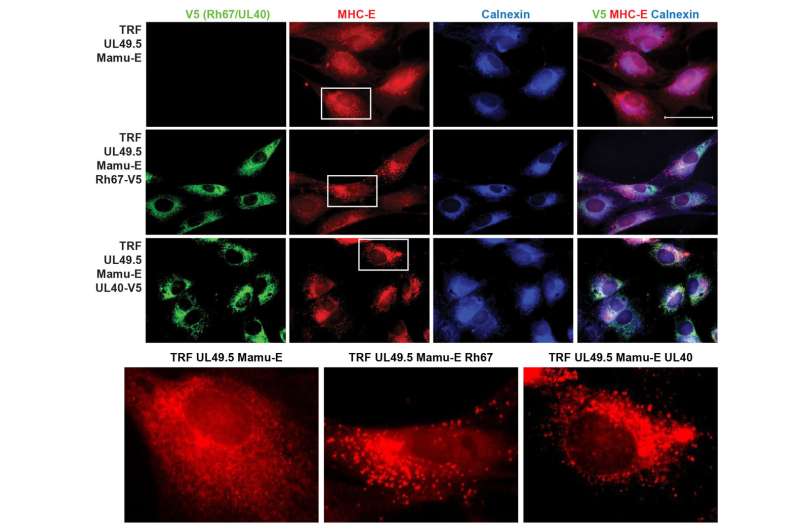
And, in a separate Science Immunology paper that was also published today, a research team led by Andrew J. McMichael, Ph.D., of Oxford University looked into whether what has been learned from nonhuman primate experiments could be transferrable to humans. These researchers showed that MHC-E-restricted CD8-positive T cells could be increased and suppress HIV in laboratory cell cultures.
This new research is being published as the OHSU Vaccine and Gene Therapy Institute celebrates the 20th anniversary of its first building opening for research in April 2001. Picker and Frueh moved to Oregon to help start the institute: Picker has led its vaccine program since its founding, and Frueh joined forces with Picker in 2006.
More information: Cytomegaloviral determinants of CD8+ T cell programming and RhCMV/SIV vaccine efficacy, Science Immunology, March 25, 2021. immunology.sciencemag.org/look … 6/sciimmunol.abg5413
Modulation of MHC-E transport by viral decoy ligands is required for RhCMV-SIV vaccine efficacy, Science, March 25, 2021. science.sciencemag.org/lookup/ … 1126/science.abe9233
HLA-E restricted Gag specific CD8+ T cells can suppress HIV-1 infection, offering vaccine opportunities, Science Immunology, March 25, 2021. immunology.sciencemag.org/look … 6/sciimmunol.abg1703