Precision medicine becomes more accessible for Australians with cancer
A new resource developed at the Garvan Institute of Medical Research and The Kinghorn Cancer Center for oncologists could help make targeted cancer therapies more accessible for Australian patients.
The TOPOGRAPH (Therapy-Oriented Precision Oncology Guidelines for Recommending Anti-cancer Pharmaceuticals) database is an online tool that catalogs oncology research to streamline the process of recommending therapeutic treatments in precision cancer medicine.
Garvan Senior Research Officer Dr. Frank Lin led the development of the platform reported this week in the journal npj Precision Oncology.
"TOPOGRAPH is uniquely useful in the Australian context because it combines up-to-date information on treatments approved for use in Australia in both clinical and trial settings," Dr. Lin says. "This tool was designed to systematically organize the vast amount of data from clinical trials and regulatory authorities into an accessible, easy to use platform for oncologists to maximize the therapeutic benefit to patients."
Bringing the data together
While several resources exist that interpret the potential therapeutic significance of genomic variations and other biomarkers in cancer, a number of factors such as government subsidies and approvals by national regulators can limit access to treatments.
Dr. Lin says that in Australia, there was a strong need for a "pragmatic, evidence-based, context-adapted tool to guide clinical management based on molecular biomarkers."
"We designed this tool because there's no good alternatives in Australia to help oncologists sieve through potential treatment options when facing a complex genomic report."
The TOPOGRAPH team, which included researchers from the Garvan Institute, The Kinghorn Cancer Center, St Vincent's Hospital, Australian Genomic Cancer Medicine Program (Omico), UNSW Sydney, The University of Sydney and the NHMRC Clinical Trials Center, conducted a comprehensive literature review and appraisal to develop a database comprising 211 predictive biomarkers, 117 cancer types and more than 400 therapies.
Oncologists can look up any of these parameters on the platform, as well as combinations of biomarkers, cancer types, and therapies to view information tailored for patients with advanced cancer.
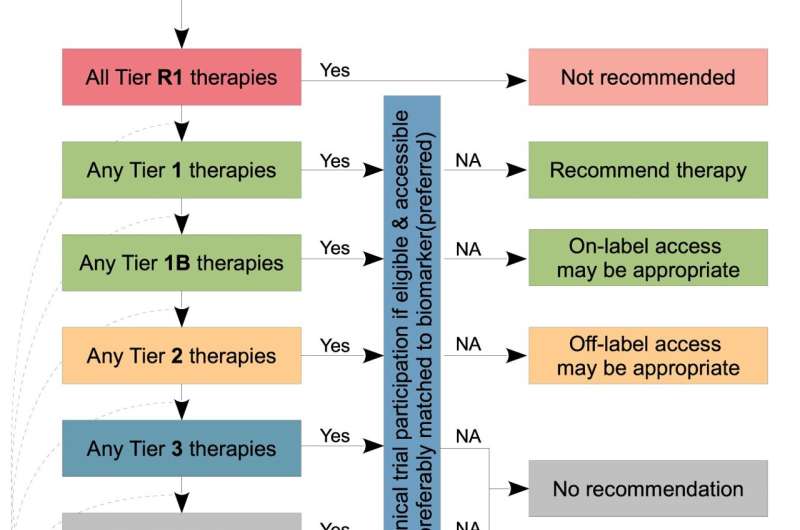
Therapies are organized into different tiers according to how effective they have been shown to be for a given cancer based on key biomarkers, its approval for use in Australia, and whether the cost of the treatment can be subsidized through the pharmaceutical benefits scheme (PBS).
Dr. Subo Thavaneswaran, Medical Oncologist at The Kinghorn Cancer Center and Garvan researcher, says the database is already proving useful in a clinical setting.
"Applying TOPOGRAPH to our Molecular Tumor Board recommendations at The Kinghorn Cancer Center gives us greater confidence in their consistency, evidence-base and understanding of accessibility to therapies in the Australian context."
Emerging research
One of the reasons TOPOGRAPH was built was to keep oncologists up to date with the latest research and treatment approvals. The researchers plan to update TOPOGRAPH with treatments and therapies as they emerge and undergo assessment by the Therapeutic Goods Administration.
Professor David Thomas, senior author of the paper and Head of the Genomic Cancer Medicine Laboratory at Garvan, Director of The Kinghorn Cancer Center and CEO of Omico, says that TOPOGRAPH could also be expanded to other jurisdictions by adjusting the platform's tier system.
"While this paper describes the use of TOPOGRAPH in the Australian context, our approach can be applied as a framework to other jurisdictions and the guidelines of different regulatory bodies. From a global oncology perspective, comparing tiered therapies between countries may help identify differences in equity of access by highlighting the disparity in drug utilization compared to scientific advances in cancer therapeutics," he says.
"In addition, there is a potential role for TOPOGRAPH to support translational research, by informing the design of new correlative studies to explore more precise biomarkers for targeted therapies."
More information: Frank P. Lin et al, Criteria-based curation of a therapy-focused compendium to support treatment recommendations in precision oncology, npj Precision Oncology (2021). DOI: 10.1038/s41698-021-00194-z