Higher rates of mutation alone are not to blame for age-related disease
New research exploring theories of aging has found that small mutations accumulating in DNA are unlikely to be fully responsible for this process.
The research, a collaboration between the Wellcome Sanger Institute, University of Birmingham, University of Edinburgh and others, found that human cells and tissues can accumulate many more mutations than are normally present, without the body showing the features associated with aging.
The new study, published today (30 September) in Nature Genetics, compared DNA taken from individuals with inherited mutations in genes involved in DNA replication with DNA from individuals who have normal versions of these genes. The researchers aimed to understand the impact of defective DNA replication on cancer risk and features associated with aging. The results suggest that build-up of mutations in normal cells is unlikely to be the only factor in the development of age-related disease, adding to the ongoing debate about the causes of aging.
One of the current models of aging suggests that accumulation of mutations in the DNA of healthy cells results in the changes that we see as the body grows older. The model is based on the observation that mutations accumulate in normal cells throughout life, and theorizes that the greater number of mutations in older people compared to younger people impairs the function of genes and disturbs cell function, ultimately leading to diseases of old-age and the visible features typically associated with aging.
However, this new research shows that human cells and tissues can function apparently normally with many more mutations than are usually present, suggesting that aging may not be due to build-up of these types of mutations alone.
DNA replication is required to duplicate the DNA in a cell ready for cell division. It involves creating an entire error-free copy of the human genome from the existing strand, and is undertaken with very high accuracy in normal healthy cells by proteins called DNA polymerases. When the DNA polymerases have a mutation, causing them to be faulty, it leads to more DNA errors, or small mutations, accumulating with each and every cell replication.
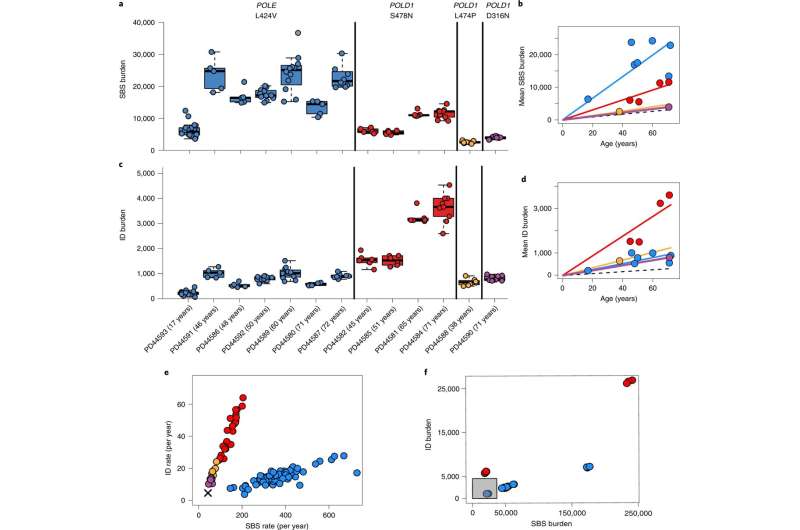
In this study, researchers from the Wellcome Sanger Institute (including the Cancer Grand Challenges Mutographs team), University of Birmingham, and University of Edinburgh, applied recently developed cutting-edge techniques to sequence the DNA of normal cells and tissues from patients who have inherited mutated versions of the DNA polymerase genes, POLE and POLD1.
By comparing tissue samples with unaffected individuals, they found that normal tissues from those who had a faulty DNA polymerase had elevated mutation rates. These study participants did not, however, show features of early onset aging or age-related diseases despite having accumulated numbers of mutations that would have made them hundreds of years old in terms of their 'mutational age." Therefore, other than an increased risk of certain cancers, the research shows that cells can accumulate many mutations and not show features associated with aging, challenging the current model.
Further research is therefore needed to understand the biological processes underlying aging.
"By focusing on people who have a known increased risk of cancer, we discovered that most or potentially all of the cells in these individuals carry an increased burden of mutations. We were amazed to see that normal and seemingly healthy cells could tolerate so many mutations. This research has given us an insight into the potential reasons for their increased risk of cancer and also offers an immensely valuable window into the process of aging. Our research shows that a higher mutational burden does not appear to result in early onset signs and features that we typically associate with aging. While other types of mutations could potentially play a role, it suggests that there is a more complex process behind aging than the accumulation of mutations alone," says Dr. Phil Robinson, co-first author and Wellcome Clinical Ph.D. Fellow at the Wellcome Sanger Institute.
"Knowing the impact of different polymerase mutations on the genetic material in cells is crucial if we are to fully understand the risk of patients with these mutations developing certain cancers and age-related disease. By showing that healthy cells can contain as many mutations as some cancer cells do, it emphasizes that there are more factors that go into making a cell cancerous than mutational burden. Further research is now necessary to understand how our findings fit into what we already know about inherited syndromes that make a person more susceptible to cancer, and if there is any way to help keep their risk of developing disease as low as possible," says Dr. Claire Palles, co-first author and Birmingham Fellow at the Institute of Cancer and Genomic Sciences at the University of Birmingham.
"The Cancer Grand Challenges Mutographs team is making incredible progress in helping us better understand the roles mutations play in tumor development. But as this study shows, the scope of the team's work goes beyond cancer and is helping us to understand more about mutations in normal tissue and even the processes of aging," says Dr. David Scott, director of Cancer Grand Challenges at Cancer Research UK.
"Understanding why our cells age and the mechanisms behind aging may help us find new ways to protect against age-related disease. This research indicates that accumulation of mutations during the course of a lifetime is unlikely, on its own, to account for the constellation of features that we term aging. Further studies are therefore required to understand what changes occurring in cells during life cause the behaviors associated with aging," Professor Sir Mike Stratton, senior author and Director of the Wellcome Sanger Institute.
More information: Philip S. Robinson et al, Increased somatic mutation burdens in normal human cells due to defective DNA polymerases, Nature Genetics (2021). DOI: 10.1038/s41588-021-00930-y
Jan Vijg et al, Pathogenic Mechanisms of Somatic Mutation and Genome Mosaicism in Aging, Cell (2020). DOI: 10.1016/j.cell.2020.06.024