Researchers find new link between a disrupted body clock and inflammatory diseases
New research from RCSI has demonstrated the significant role that an irregular body clock plays in driving inflammation in the body's immune cells, with implications for the most serious and prevalent diseases in humans.
Published in Frontiers in Immunology, the research was led by the School of Pharmacy and Biomolecular Sciences at RCSI University of Medicine and Health Sciences.
The circadian body clock generates 24-hour rhythms that keep humans healthy and in time with the day/night cycle. This includes regulating the rhythm of the body's own (innate) immune cells called macrophages. When these cell rhythms are disrupted (due to things like erratic eating/sleeping patterns or shift work), the cells produce molecules which drive inflammation. This can lead to chronic inflammatory diseases such as heart disease, obesity, arthritis, diabetes and cancer, and also impact our ability to fight infection.
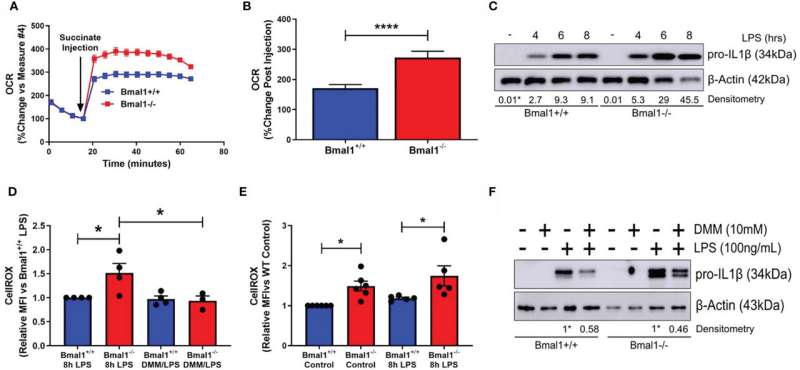
In this study, the researchers looked at these key immune cells called macrophages with and without a body clock under laboratory conditions. They were interested to understand if macrophages without a body clock might use or 'metabolise' fuel differently, and if that might be the reason these cells produce more inflammatory products.
The researchers found that macrophages without a body clock took up far more glucose and broke it down more quickly than normal cells. They also found that, in the mitochondria (the cells energy powerhouse), the pathways by which glucose was further broken down to produce energy were very different in macrophages without a clock. This led to the production of reactive oxygen species (ROS) which further fuelled inflammation.
Dr. George Timmons, lead author on the study, said: "Our results add to the growing body of work showing why disruption of our body clock leads to inflammatory and infectious disease, and one of the aspects is fuel usage at the level of key immune cells such as macrophages."
Dr. Annie Curtis, senior lecturer at RCSI School of Pharmacy and Biomolecular Sciences and senior author on the paper, added: "This study also shows that anything which negatively impacts on our body clocks, such as insufficient sleep and not enough daylight, can impact on the ability of our immune system to work effectively."
More information: George A. Timmons et al, The Circadian Clock Protein BMAL1 Acts as a Metabolic Sensor In Macrophages to Control the Production of Pro IL-1β, Frontiers in Immunology (2021). DOI: 10.3389/fimmu.2021.700431